Creating a Study or Submission
Overview: Creating a Study or Submission
You create a study or
submission by providing basic object metadata such as name, description,
and content location in the metadata tree. Then, SAS Clinical Data
Integration collects metadata about the item. For example, a study
collects metadata such as protocol title, indication, and phase. After
metadata is collected, the versions of the data standards that can
be used for the study or submission are defined.
Note: Only an administrator can
set the default content for a study or submission. For more
information, see Adding Users to the Clinical Administrators Group.
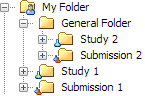
Folder Organization of Studies and Submissions
A study or submission
can be located at the root of the hierarchy in the Folders tree
(Study 1 and Submission 1 in the following figure) or within a general
folder (Study 2 and Submission 2).
You can create more
complex hierarchies based on the containment rules shown in the following
table:
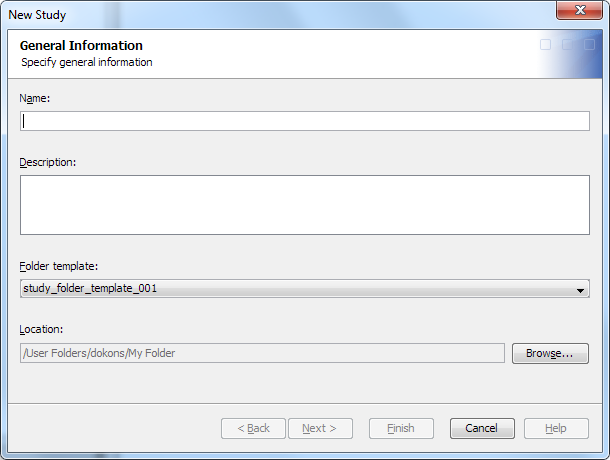
Create a Study or Submission
To create a study or
submission, perform the following steps:
-
The name must meet certain requirements. (See Name Requirements. )The description must meet certain requirements. (See Description Requirements.)
-
For information about the location of a study or submission, see Folder Organization of Studies and Submissions.
-
The libraries that are available on this page are predetermined by the default content for a study or submission. For more information, see Working with Library Templates.
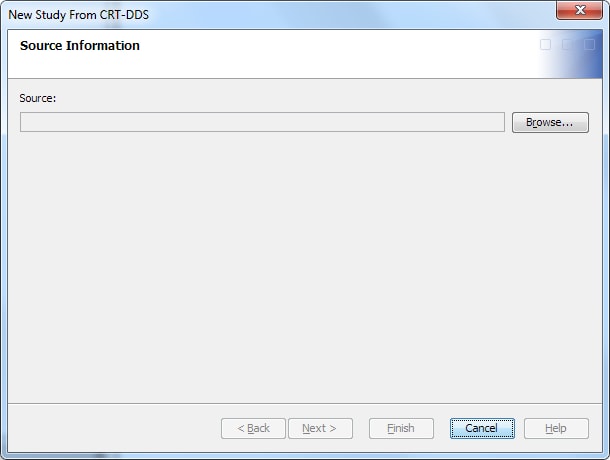
Create a Study from a CRT-DDS define.xml File
To create a study from
a study definition in a CRT-DDS define.xml file, perform the following
steps:
-
Note: The name and description are automatically derived from the define.xml file. However, you can change the values.The name must meet certain requirements. (See Name Requirements. )The description is not required. If you provide a description, it must meet certain requirements. (See Description Requirements.)
-
For information about the location of a study, see Folder Organization of Studies and Submissions.
-
The libraries that appear on this page are predetermined by the default content of a study. For more information, see Working with Library Templates.
Copyright © SAS Institute Inc. All rights reserved.