Creating a Study or Submission
Overview: Creating a Study or Submission
You create a study or
submission by providing basic object metadata such as name, description,
and content location in the metadata tree. Then, SAS Clinical Data
Integration collects metadata about the item. For example, a study
collects metadata such as protocol title, indication, and phase. After
metadata is collected, the versions of the data standards that can
be used for the study or submission are defined.
Note: Only a clinical administrator
can set the default content for a study or submission. For more
information, see
The Clinical Administrator Group.
You can create a study
from a study definition in a define.xml file. If the define.xml file
contains multiple study definitions, only the first study definition
is imported.
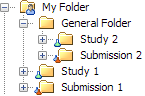
Folder Organization of Studies and Submissions
A study or submission
can be located at the root of the hierarchy in the Folders tree
(Study 1 and Submission 1 in the following figure) or within a general
folder (Study 2 and Submission 2).
You can create more
complex hierarchies based on the containment rules shown in the following
table:
|
Content
|
|||
|
Study
|
Submission
|
||
|
Container
|
Study
|
not allowed
|
not allowed
|
|
Submission
|
allowed
|
not allowed
|
|
Using the containment
rules, here is an example of a more complex folder hierarchy:
Create a Study or Submission
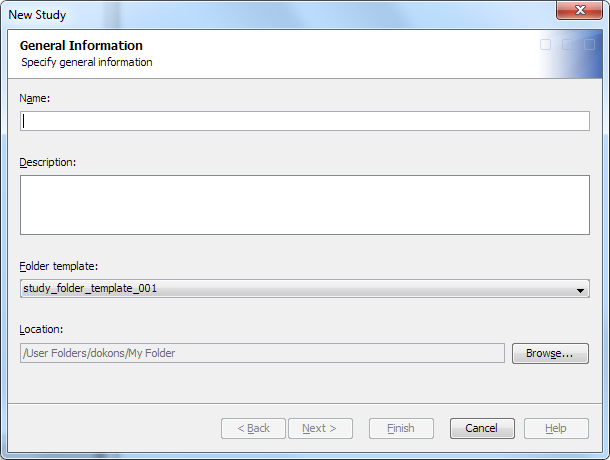
To create a study or
submission, perform the following steps:
-
The name must meet certain requirements. (See Name Requirements. )The description must meet certain requirements. (See Description Requirements.)
-
For more information, see Working with Folder Templates.
-
For information about the location of a study or submission, see Folder Organization of Studies and Submissions.
-
The properties that appear on this page and their default values are predetermined by the administrator's configuration of the data standard’s property model.Note: Do not use single quotation marks, double quotations marks, or hyphens in the property value fields.
-
The libraries that are available on this page are predetermined by the default content for a study or submission. For more information, see Working with Library Templates.
Create a Study from a Define.xml File
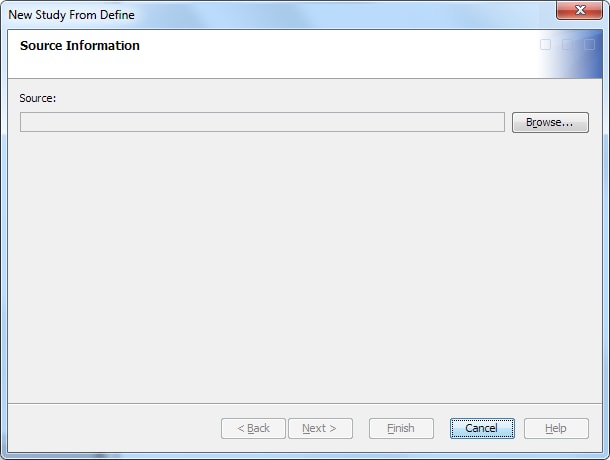
To create a study from
a study definition in a define.xml file, perform the following steps:
-
Note: The name and description are automatically derived from the define.xml file. However, you can change the values.The name must meet certain requirements. (See Name Requirements. )The description is not required. If you provide a description, it must meet certain requirements. (See Description Requirements.)
-
For more information, see Working with Folder Templates.
-
For information about the location of a study, see Folder Organization of Studies and Submissions.
-
The properties that appear on this page and their default values are predetermined by the administrator’s configuration of the data standard’s property model.The attribute values that are specified in the define.xml file and that map to SAS Clinical Data Integration properties are imported.Note: Do not use single quotation marks, double quotations marks, or hyphens in the property value fields.
-
The libraries that appear on this page are predetermined by the default content of a study. For more information, see Working with Library Templates.
Copyright © SAS Institute Inc. All rights reserved.