The SEQTEST Procedure
- Overview
- Getting Started
-
Syntax

-
Details
 Input Data Sets Boundary Variables Information Level Adjustments at Future Stages Boundary Adjustments for Information Levels Boundary Adjustments for Minimum Error Spending Boundary Adjustments for Overlapping Lower and Upper beta Boundaries Stochastic Curtailment Repeated Confidence Intervals Analysis after a Sequential Test Available Sample Space Orderings in a Sequential Test Applicable Tests and Sample Size Computation Table Output ODS Table Names Graphics Output ODS Graphics Acknowledgments
Input Data Sets Boundary Variables Information Level Adjustments at Future Stages Boundary Adjustments for Information Levels Boundary Adjustments for Minimum Error Spending Boundary Adjustments for Overlapping Lower and Upper beta Boundaries Stochastic Curtailment Repeated Confidence Intervals Analysis after a Sequential Test Available Sample Space Orderings in a Sequential Test Applicable Tests and Sample Size Computation Table Output ODS Table Names Graphics Output ODS Graphics Acknowledgments -
Examples
 Testing the Difference between Two Proportions Testing an Effect in a Regression Model Testing an Effect with Early Stopping to Accept H0 Testing a Binomial Proportion Comparing Two Proportions with a Log Odds Ratio Test Comparing Two Survival Distributions with a Log-Rank Test Testing an Effect in a Proportional Hazards Regression Model Testing an Effect in a Logistic Regression Model
Testing the Difference between Two Proportions Testing an Effect in a Regression Model Testing an Effect with Early Stopping to Accept H0 Testing a Binomial Proportion Comparing Two Proportions with a Log Odds Ratio Test Comparing Two Survival Distributions with a Log-Rank Test Testing an Effect in a Proportional Hazards Regression Model Testing an Effect in a Logistic Regression Model - References
| Input Data Sets |
The BOUNDARY= data set option is required, and if neither the DATA= nor the PARMS= data set option is specified, the procedure derives statistics such as Type I and Type II error probabilities from the BOUNDARY= data set. The resulting boundaries are displayed with the scale specified in the BOUNDARYSCALE= option.
BOUNDARY= SAS Data Set
The BOUNDARY= data set provides the boundary information for the sequential test. At stage  , the data set is usually created with an ODS OUTPUT statement from the "Boundary Information" table created by the SEQDESIGN procedure. At each subsequent stage, the data set is usually created with an ODS OUTPUT statement from the "Test Information" table that was created by the SEQTEST procedure at the previous stage. See the section Getting Started: SEQTEST Procedure for an illustration of the BOUNDARY= data set option.
, the data set is usually created with an ODS OUTPUT statement from the "Boundary Information" table created by the SEQDESIGN procedure. At each subsequent stage, the data set is usually created with an ODS OUTPUT statement from the "Test Information" table that was created by the SEQTEST procedure at the previous stage. See the section Getting Started: SEQTEST Procedure for an illustration of the BOUNDARY= data set option.
The BOUNDARY= data set contains the following variables:
_Scale_, the boundary scale, with the value MLE for the maximum likelihood estimate, STDZ for the standardized Z, SCORE for the score statistic, or PVALUE for the nominal
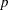 -value. Note that for a two-sided design, the nominal
-value. Note that for a two-sided design, the nominal 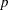 -value is the one-sided fixed-sample
-value is the one-sided fixed-sample 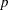 -value under the null hypothesis with a lower alternative hypothesis.
-value under the null hypothesis with a lower alternative hypothesis. _Stop_, the stopping criterion, with the value REJECT for rejecting the null hypothesis
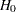 , ACCEPT for accepting
, ACCEPT for accepting 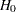 , or BOTH for both rejecting and accepting
, or BOTH for both rejecting and accepting 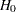
_ALT_, the type of alternative hypothesis, with the value UPPER for an upper alternative, LOWER for a lower alternative, or TWOSIDED for a two-sided alternative
_Stage_, the stage number
the boundary variables, a subset of Bound_LA for lower
 boundary, Bound_LB for lower
boundary, Bound_LB for lower 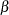 boundary, Bound_UB for upper
boundary, Bound_UB for upper 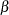 boundary, and Bound_UA for upper
boundary, and Bound_UA for upper  boundary
boundary AltRef_L, the lower alternative reference, if ALT=LOWER or ALT=TWOSIDED
AltRef_U, the upper alternative reference, if ALT=UPPER or ALT=TWOSIDED
_InfoProp_, the information proportion at each stage
Optionally, the BOUNDARY= data set also contains the following variables:
_Info_, the information level at each stage
NObs, the required number of observations for nonsurvival data at each stage
Events, the required number of events for survival data at each stage
Parameter, the variable specified in the DATA(TESTVAR=) or PARMS(TESTVAR=) option
Estimate, the parameter estimate
If the BOUNDARY= data set contains the variable Parameter for the test variable that is specified in the TESTVAR= option, and the variable Estimate for the test statistics, then these test statistics are also displayed in the output test information table and output test plot.
DATA < (TESTVAR= variable) > = SAS Data Set
The DATA= data set provides the test variable information for the current stage of the trial. Such data sets are usually created with an ODS OUTPUT statement by using a procedure such as PROC MEANS. See Testing a Binomial Proportion for an illustration of the DATA= data set option.
The DATA= data set includes the following variables:
_Stage_, the stage number
_Scale_, the scale for the test statistic, with the value MLE for the maximum likelihood estimate, STDZ for the standardized Z, SCORE for the score statistic, or PVALUE for the nominal
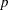 -value
-value _Info_, the information level
NObs, the number of observations for nonsurvival data at each stage
Events, the number of events for survival data at each stage
test variable, specified in the TESTVAR= option, contains the test variable value in the scale specified in the _Scale_ variable
With the specified DATA= data set, the procedure derives boundary values from the information levels in the _Info_ variable. If the data set does not include the _Info_ variable, then the information levels are derived from the NObs or Events variable in the DATA= data set if the variable is also in the input BOUNDARY= data set. That is, the information level at stage 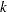 is computed as
is computed as  , where
, where 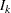 and
and 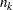 are the information level and sample size at stage
are the information level and sample size at stage 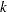 in the BOUNDARY= data set and
in the BOUNDARY= data set and 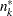 is the sample size at stage
is the sample size at stage 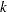 in the DATA= data set. Otherwise, the information levels from the BOUNDARY= data set are used.
in the DATA= data set. Otherwise, the information levels from the BOUNDARY= data set are used.
If the TESTVAR= option is specified, the DATA= data set must also include the test variable for the test statistic and _Scale_ variable for the corresponding scale. Note that for a two-sided design, the nominal 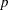 -value is the one-sided fixed-sample
-value is the one-sided fixed-sample 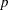 -value under the null hypothesis with a lower alternative hypothesis.
-value under the null hypothesis with a lower alternative hypothesis.
PARMS < (TESTVAR= variable) > = SAS Data Set
The PARMS= data set provides a parameter estimate and associated standard error for the current stage of the trial. Such data sets are usually created with an ODS OUTPUT statement by using procedures such as the GENMOD, GLM, LOGISTIC, and REG procedures. See the section Getting Started: SEQTEST Procedure for an illustration of the PARMS= data set option.
The PARMS= data set includes the following variables:
_Stage_, the stage number
_Scale_, the scale for the test statistic, with the value MLE for the maximum likelihood estimate, STDZ for the standardized Z, SCORE for the score statistic, or PVALUE for the nominal
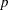 -value
-value Parameter, Effect, Variable, or Parm, which contains the variable specified in the TESTVAR= option
Estimate, the parameter estimate
StdErr, standard error of the parameter estimate
With the specified PARMS= data set, the information level is derived from the StdErr variable. For a score statistic, the information level 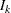 is the variance of the statistic,
is the variance of the statistic,  , where
, where  is the standard error in the StdErr variable. Otherwise, the information level is the inverse of the variance of the statistic,
is the standard error in the StdErr variable. Otherwise, the information level is the inverse of the variance of the statistic,  . If the data set does not include the StdErr variable, the information levels derived from the BOUNDARY= data set are used.
. If the data set does not include the StdErr variable, the information levels derived from the BOUNDARY= data set are used.
If the TESTVAR= option is specified, the PARMS= data set also includes the variable Parameter, Effect, Variable, or Parm for the test variable, Estimate for the test statistic, and _Scale_ variable for the corresponding scale. Note that for a two-sided design, the nominal 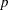 -value is the one-sided fixed-sample
-value is the one-sided fixed-sample 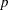 -value under the null hypothesis with a lower alternative hypothesis.
-value under the null hypothesis with a lower alternative hypothesis.