Sample Reporting Methodology
Overview
The primary purpose
of the CDISC ADaM standard is to build analysis data sets that support
analysis and reporting of clinical research. This purpose, in turn,
supports the greater goal of submitting clinical research results
to regulatory authorities. These regulatory authorities determine
the efficacy and safety of a medical device or product.
The Analysis Data Model,
Version 2.1, ADaM Document provides specifications for the structure
and content of analysis data sets, and a suggested metadata format
for documenting the analysis results generated. Analysis results metadata
describe the major attributes of a specified analysis result found
in a clinical study report or submission. Analysis results metadata
support traceability from an analysis result used in a statistical
display to the data in the analysis data sets.
The SAS Clinical Standards
Toolkit representation of the ADaM standard includes a sample implementation
of an analysis reporting methodology.
Note: This methodology is for illustrative
purposes only. Each organization has its own set of processes and
workflows that support the generation of a clinical study report or
submission. The sample reporting methodology provided with the SAS
Clinical Standards Toolkit is intended to be representative of similar
industry reporting methodologies. The intent is not to provide a definitive
reporting methodology, but to illustrate the interaction of reporting
components through the adoption of the ADaM standard.
Key Clinical Trial Reporting Components
|
Defined by the Analysis
Data Model, Version 2.1, ADaM Document, Section 5.3. For more information,
see Analysis Results Metadata.
|
|
The majority of the
files supporting the ADaM sample reporting methodology provided with
the SAS Clinical Standards Toolkit are located in the ADaM analysis
folder:
-
The code folder contains the code to create each statistical display. This corresponds to the Analysis Results component described in Key Clinical Trial Reporting Components.
-
The data folder contains the display-specific metadata noted in the TLF Metadata component of Key Clinical Trial Reporting Components.
-
The documents folder contains table shells for the TLF Metadata component. For more information about table shells, seeTLF Metadata.
TLF Metadata
A common industry reporting
strategy is to create table shells (templates)
which specify the output for each statistical display. The SAS Clinical
Standards Toolkit provides sample table shells in this file:
!sasroot /../SASClinicalStandardsToolkitADaM21/1.4/sample/cdisc-adam-2.1/sascstdemodata/analysis/documents/Mock_tables_shells.pdfThe elements of each
table shell (for example, titles, footnotes, headings, column and
row labels, cell formatting, and so on) are sometimes captured in
a metadata format, often in Microsoft Excel files. The usual intent
is to create reporting macros that can generate analysis reports based
on this metadata, so that changes in metadata are all that is required
to modify and rerun any report.
For the SAS Clinical
Standards Toolkit 1.4, sample metadata is included that illustrates
the use of such metadata within the ADaM reporting environment.
Note: The sample metadata provided
does not represent a full implementation. All metadata fields used
in the report examples are not provided.
!sasroot /../SASClinicalStandardsToolkitADaM21/1.4/sample/cdisc-adam-2.1/sascstdemodata/metadata/tlfddt.xmlTo interpret this metadata,
a sample SAS XML map file (tlfddt.map) is provided in the same folder.
SAS data sets, representing this XML metadata, are provided in the
library of SAS files located in this folder:
!sasroot /../SASClinicalStandardsToolkitADaM21/1.4/sample/cdisc-adam-2.1/sascstdemodata/analysis/dataThe following figures
provide examples of some of the metadata available in the source XML
file. This metadata has been extracted into SAS data sets.
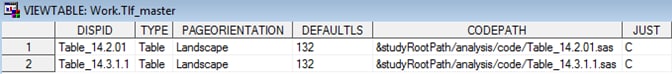
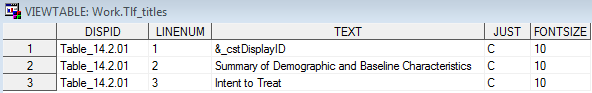
Sample TLF Metadata: Tlf_index
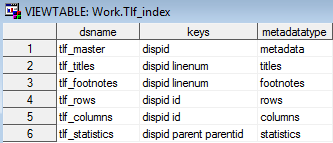
Row 1 of the Tlf_master
data set describes a centered landscape table and shows where the
generating code can be found. The title for that table is provided
in the Tlf_titles file. These tables correspond
to the table shell titles specified in SAS Clinical Standards Toolkit Sample Table Shell.
Analysis Programs
!sasroot /../SASClinicalStandardsToolkitADaM21/1.4/sample/cdisc-adam-2.1/sascstdemodata/analysis/codeAs noted above, these
sample analysis programs do not fully use the sample TLF metadata
provided with the SAS Clinical Standards Toolkit. The basic coding
strategy adopted with each SAS Clinical Standards Toolkit sample analysis
program is to build each section (one or more row combinations) and
to concatenate these sections into a single input file used by PROC
REPORT.
A sample driver module
is provided to perform the process setup, to define (or reference)
the sasreferences data set, to perform any required report setup,
and to call the generic ADaM reporting macro adam_createdisplay().
This sample driver module is located in this folder:
!sasroot /../SASClinicalStandardsToolkitADaM21/1.4/sample/cdisc-adam-2.1/sascstdemodata/programs/analyze_data.sasIn the sample driver
module, a call is made to adam_createdisplay() for each analysis report
to be produced:
%adam_createdisplay (displaysrc=Metadata,useanalysisresults=N,usetlfddt=Y, displayid=%str(Table_14.2.01));
To automate this process
of creating all analysis reports for a study, it would be necessary
to cycle through any available metadata (such as that described
in Sample TLF Metadata: Tlf_master) to construct multiple calls to the adam_createdisplay macro. The
adam_createdisplay macro header provides an overview of the macro
functionality and a summary of the defined macro parameters:
adam_createdisplay
Generate an analysis result from ADaM analysis data sets
The basic function of this code module is to create an analysis result display. The path to
the code to create the display is provided either directly in the macro parameters or is
derived from a metadata source such as the analysis results metadata or Tables, Listings
and Figures data definition metadata (TLFDDT) you maintain and reference in the SASReferences
data set.
Two primary paths (parameter settings) are supported:
(1) A code source is specified. A fully qualified path is required. The expectation is
that this module will be %included below to generate an analysis result (display).
(2) Metadata is to be used to provide the information necessary to generate an analysis
result (display). This metadata will be in the form of the CDISC-ADaM Analysis Results metadata,
supplemental Tables, Listings and Figures data definition metadata (TLFDDT), or both.
@param displaysrc - Where will information come from to generate result? Values: Code |
Metadata (default). Required.
@param displaycode - Either a valid filename or the fully qualified path to code to produce an
analysis result. Required and used only if displaysrc=Code. All of the remaining parameters
below are ignored.
@param useanalysisresults - Should the study-specific analysis results metadata be used to
provide report metadata? Values: N | Y (default). Either this parameter or usetlfddt must
be set to Y if displaysrc=Metadata. If both the useanalysisresults and usetlfddt parameters
are set to Y, useanalysisresults will take precedence.
@param usetlfddt - Should the study-specific mock tables shells metadata (known here as Tables,
Listings and Figures data definition metadata (TLFDDT)) be used to provide report metadata?
Values: N | Y (default). Either this parameter or useanalysisresults must be set to Y if
displaysrc=Metadata. If both the useanalysisresults and usetlfddt parameters are set to Y,
useanalysisresults will take precedence.
@param displayid - The ID of the display from the designated metadata source. Required if
displaysrc=Metadata.
@param displaypath - Either a valid filename or the fully qualified path to the generated
display. Optional. If not provided, code looks in SASReferences for type=report.
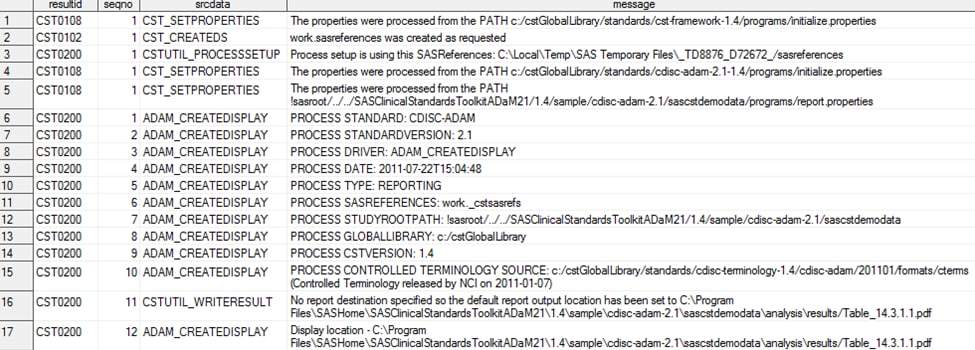
The SAS Clinical Standards
Toolkit ADaM reporting methodology uses a report.properties file to
specify the default report format. By default, the property (and global
macro variable) _cstDefaultReportFormat is set to PDF. Submitting
the analyze_data.sas driver module produces the specified statistical
displays and generates a process results data set. Here is a sample
results data set:
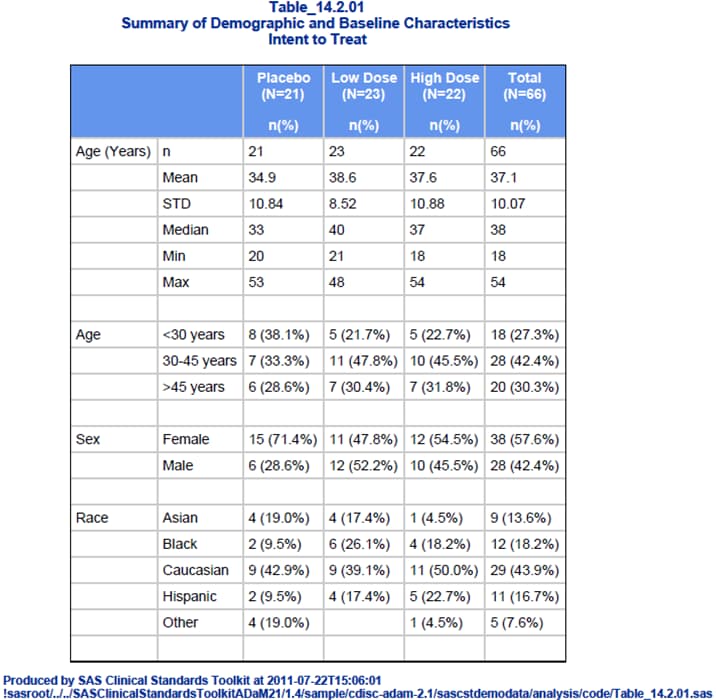
Analysis Results (Tables, Listings, and Figures)
Each generated statistical
display should correspond to a table shell, as described in the TLF
Metadata section. (See SAS Clinical Standards Toolkit Sample Table Shell.)
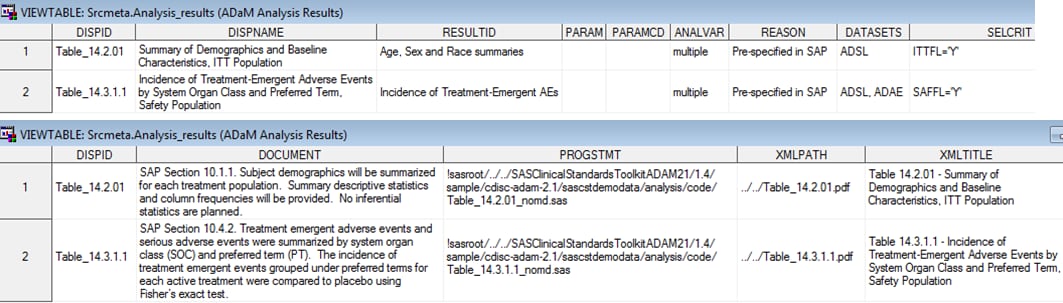
Analysis Results Metadata
The Analysis Data Model,
Version 2.1, ADaM Document provides specifications for capturing analysis
results. As a result, traceability back to the contributing source
data is possible. Analysis Results Metadata identifies the columns to be included in the analysis results
data set. All analysis results metadata for the
two statistical displays provided with the SAS Clinical Standards
Toolkit is shown in this figure: