Validation of ADaM Data Sets
Overview
Validation of CDISC
ADaM data sets in the SAS Clinical Standards Toolkit uses the same
validation methodology used for other standards. Within the global
standards library, registering each standard includes setting the
flag supportsvalidation in the metadata standards data set. All standards
that support validation, including ADaM, use the same validation framework
and processes described in Validation.
ADaM validation of ADSL
and BDS data sets is based on the CDISC ADaM Validation
Checks Version 1.1 Maintenance Release (dated and released
January 21, 2011 to correct errors and remove duplicate checks). This
documentation was prepared by the CDISC ADaM team. The version 1.1
release identifies 173 validation checks to be performed. The SAS
Clinical Standards Toolkit defines validation checks using a combination
of two files:
Each of these ADaM data
sets has only 159 records. Fourteen validation checks have been combined
with other checks by the SAS Clinical Standards Toolkit.
Checks 92 and 93 are
defined and run together as check ADAM0092 because the check macro
that is used (cstcheck_notunique) checks both conditions by default.
The SAS Clinical Standards Toolkit supports all of the checks specified
in the version 1.1 release. These checks are
listed in Validation Checks.
Specific Check Implementation Details
CDISC ADaM Validation Check Implementation Details
Unique Validation Properties
Validation Check Macros
* These macros are new
to the SAS Clinical Standards Toolkit 1.4. They are used only for
CDISC ADaM validation, although they are available to all standards.
Note: This list represents a subset
of check macros that are available to all standards to be validated.
For information
about the purpose and use of each check macro, see Macro Application Programming Interface.
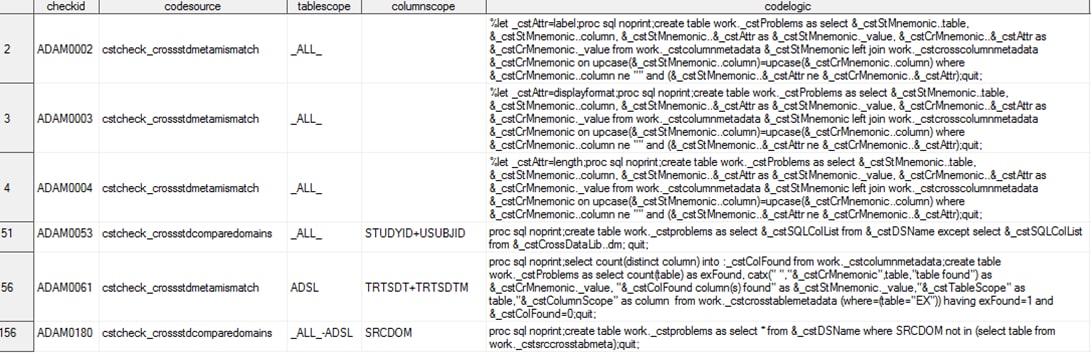
Cross-Standard Validation Checks
Six ADaM validation
checks require a comparison of ADaM data or metadata with SDTM data
or metadata. These checks require the availability of table and column
metadata from two different standards. To support this comparison,
two new check macros (cstcheck_crossstdcomparedomains and cstcheck_crossstdmetamismatch)
are available in the SAS Clinical Standards Toolkit 1.4. Part of the metadata
available in the Validation Master data set for the six ADaM cross-standard
validation checks is shown in Partial Metadata for the CDISC ADaM Cross-Standard Validation Checks.
Sample Data for Validation and Reporting
The SAS Clinical Standards
Toolkit implementation of ADaM includes two sets of data and metadata.
One set supports the SAS Clinical Standards Toolkit ADaM reporting.
In this set, few, if any, data errors and anomalies are included,
and this set is considered a clean, analysis-ready set of data. A
second set includes illustrative data and metadata errors to demonstrate
ADaM validation functionality.
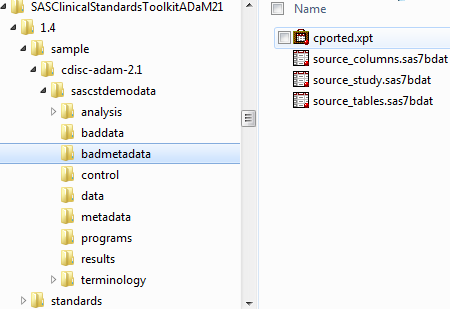
The following figure
shows some of the installed SAS files for ADaM, the data and metadata
folders that support reporting, and the baddata and badmetadata folders
that support validation. The corresponding sample driver modules (analyze_data.sas
and validate_data.sas, respectively), which are located in the programs
folder (as shown in Example Folder Hierarchy for a CDISC ADaM Sample Study) point to the
correct source data and metadata folders.
Example Folder Hierarchy for a CDISC ADaM Sample Study
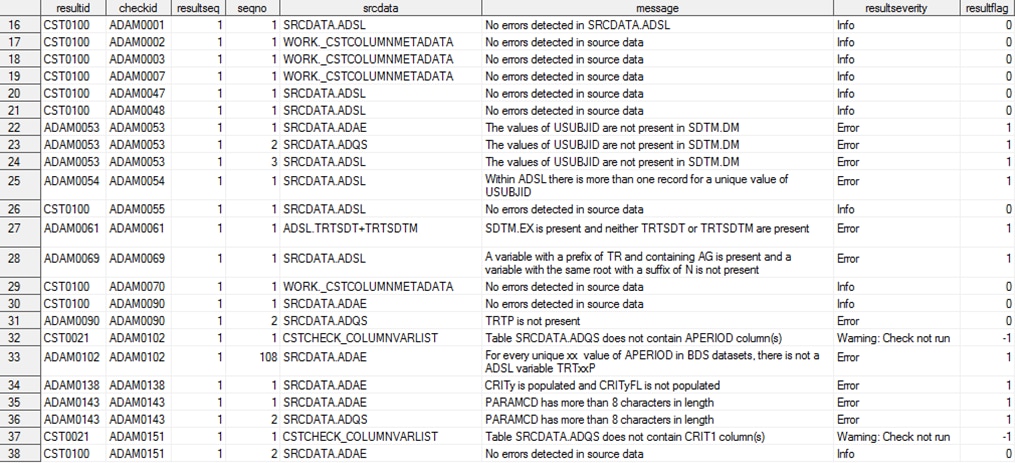
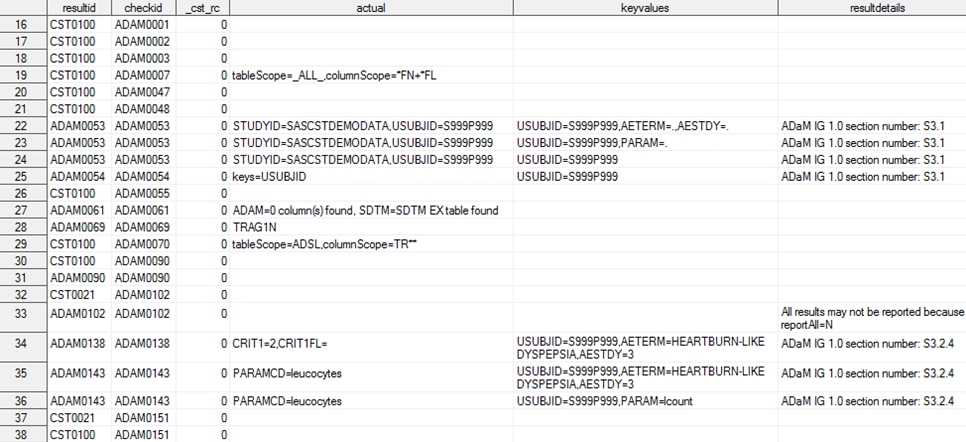
Validation Results
The results of an
ADaM validation process, as documented in the validation_results data
set, are shown in Results from an ADaM Validation Process (Partial Listing) and Results from an ADaM Validation Process (Partial Listing—Continued). The first 15 records of the data set have been excluded
from the display because they report generic process setup and metadata
information common to all validation processes.
Records 22 through 24
report the results of one of the cross-standard validation checks.
This validation check finds a subject (USUBJID) in the ADaM data sets
that was not found in the SDTM DM domain.
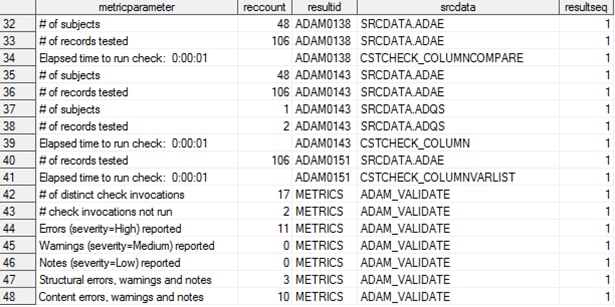
A partial report of
the validation_metrics data set (including a process summary noting
that 17 checks were attempted, two could not be run, and 11 errors
were detected) is shown in Metrics from an ADaM Validation Process (Partial Listing). The two checks that could not be run referenced columns
in the check metadata that could not be found or assessed in the source
data sets.