SAS Representation of CDISC ADaM Metadata
The SAS Clinical Standards
Toolkit provides a SAS metadata representation of each supported standard.
The SAS Clinical Standards Toolkit implementation of the CDISC ADaM
2.1 standard provides an interpretation of Analysis Data Model, Version
2.1, ADaM document and the Analysis Data Model Implementation
Guide, Version 1.0. The Analysis Data Model identifies
four types of ADaM metadata that are captured and supported by the
SAS Clinical Standards Toolkit.
In the SAS Clinical
Standards Toolkit, the Analysis data set metadata is captured in the
reference_tables and class_tables data sets, which are located here:
The SAS Clinical Standards
Toolkit captures more metadata than might be specified for a standard.
This helps support SAS Clinical Standards Toolkit functionality and
provides greater consistency across supported standards.
This table provides
the mapping of the Analysis data set metadata defined by the CDISC
ADaM team to the SAS metadata representation in the reference_tables
data set:
Analysis Data Set Metadata
**Source: Version 2.1
of the Analysis Data Model Document, Section 5.1, Analysis Dataset
Metadata, Table 5.1.1
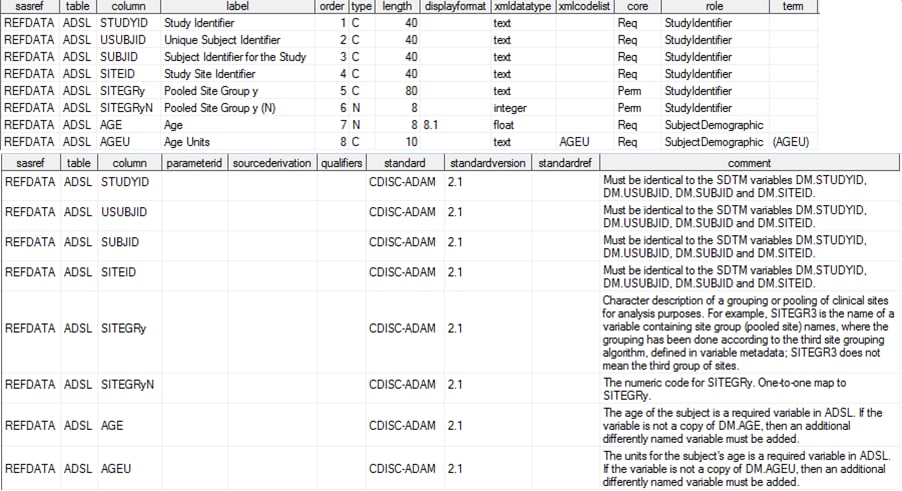
The reference_tables
data set provided with the SAS Clinical Standards Toolkit 1.4 contains
three records for the ADaM ADSL Analysis data set, a representative
ADaM BDS data set, and an ADaM analysis results (RESULTS) data set.
CDISC ADaM specifies that only the ADSL data set is required. Any
number of BDS data sets can be defined as required for each study.
A single, optional analysis results data set can be used for each
study. Sample Reference_Tables Record (CDISC ADaM 2.1) lists the column contents for the ADSL data set record
in the reference_tables data set.
In the SAS Clinical
Standards Toolkit, Analysis Variable metadata is captured in the reference_columns
and class_columns data sets in the global standards library folder:
This table provides
the mapping of Analysis Variable metadata defined by the CDISC ADaM
team to the SAS metadata representation in the reference_columns data
set:
Analysis Variable Metadata
**Source: Analysis Data
Model, Version 2.1, ADaM Document, Section 5.2, Analysis Variable
Metadata, Table 5.2.1
The reference_columns
data set provided with the SAS Clinical Standards Toolkit 1.4 contains
one record for each column in each of the three data sets (ADSL, BDS,
and RESULTS) in the reference_tables data set. This results in 63
records (columns) for ADSL, 142 records (columns) for BDS, and 13
records (columns) for the RESULTS data set.
Core reference_columns
metadata for each column is in the Analysis Data Model
Implementation Guide, Version 1.0. ADSL Columns as Specified in the Analysis Data Model Implementation Guide provides an excerpt of ADSL column metadata as itemized
in Table 3.1.1 of the Analysis Data Model Implementation
Guide, Version 1.0. This metadata has
been translated into the SAS representation of ADSL as shown in ADSL Columns as Defined in reference_columns Data Set. (See also Sample Reference_Columns Record (CDISC ADaM 2.1), which provides
the full set of column metadata for the BDS TRTP column in the reference_columns
data set.)
The SAS representation
of ADaM analysis metadata in reference_tables and reference_columns
provides a study template based on the Data Model Document and the Analysis Data Model Implementation Guide, Version 1.0. Each specific study implementation of ADaM creates multiple BDS
data sets. The number of data sets is determined by the study design,
the statistical analysis plan, and the available source data (for
example, SDTM). Each analysis data set (including ADSL) might contain
a different subset of columns defined by the CDISC ADaM model.
The SAS implementation
makes assumptions about the data type and length of each column. These
assumptions represent a typical implementation consistent with SDTM
metadata and conventions for specific types of columns. For example,
most identifiers have a default length of 40, most flags have a length
of 1, and columns using controlled terminology are defined with a
length that is long enough to capture the longest controlled term.
A third type of metadata
identified in the Analysis Data Model, Version 2.1, ADaM Document
(see ADaM Document Sources for Each Metadata Type) is analysis
parameter value-level metadata. As noted in the ADaM document:
“Each BDS data
set can contain multiple analysis parameters. In a BDS analysis dataset,
the variable PARAM contains a unique description for every analysis
parameter included in that dataset. Each value of PARAM identifies
a set of one or more rows in the dataset. To describe how variable
metadata vary by PARAM/PARAMCD, the metadata element PARAMETER IDENTIFIER
is required in variable-level metadata for a BDS analysis dataset.
This PARAMETER IDENTIFIER metadata element identifies which variables
have metadata that vary depending on PARAM/PARAMCD, and links the
metadata for a variable to the appropriate value of PARAM/PARAMCD.”
The reference_columns
data set contains a column named parameterid that can be used to capture
the value-level metadata for BDS data sets. For more information about
analysis parameter value-level metadata, see sections 5.2.1 and 5.2.2
of the Analysis Data Model, Version 2.1, ADaM Document.
The final set of metadata
prescribed by the Analysis Data Model, Version 2.1, ADaM Document
is analysis results metadata. Analysis results metadata is described
in the ADaM document:
“These metadata
provide traceability from a result used in a statistical display to
the data in the analysis data sets. Analysis results metadata are
not required. Analysis results metadata describe the major attributes
of a specified analysis result found in a clinical study report or
submission.”
The metadata fields
used to describe an analysis result are listed in Analysis Results Metadata. In the SAS Clinical Standards Toolkit, these metadata fields
are captured in the reference_columns data set (where table=’RESULTS’),
and serve as a template to initialize an analysis results data set. For more information,
see ADaM Data Set Templates.
Analysis Results Metadata