Creating a Define-XML 2.0 define.xml File from SDTM Source Data
Assumption
You have a library of
CDISC SDTM SAS data sets (which are not read by this process) from
which a set of metadata (in the form of source_study, source_tables,
source_columns, source_codelists, source_values, and source_documents
data sets) has been created. This metadata must contain the expected,
correctly typed columns created for the sample study provided by SAS.
The source_study, source_tables, and source_columns data sets are
required to create a Define-XML 2.0 define.xml file.
Step 1: Extract Available SDTM Source Metadata into the SAS Representation of Define-XML 2.0 Metadata
The initial task is
to extract the available SDTM metadata into Define-XML 2.0 metadata
files. The SAS representation of Define-XML 2.0 involves 46 data sets,
but only 31 of these are typically used for the creation of a Define-XML
2.0 file. The other 15 data sets contain ODM (Operational Data Model)
metadata that is an extension of the Define-XML 2.0 model. The sample
driver create_sasdefine_from_source.sas, modified to point to your
specific SDTM study metadata, must be submitted to create the SAS
representation of Define-XML 2.0 metadata. This process builds the
31 core data sets, but it might not populate all of them (depending
on the completeness of the metadata of your study).
The sample driver runs
this macro:
%define_sourcetodefine(
_cstOutLib=srcdata,
_cstSourceStudy=sampdata.source_study,
_cstSourceTables=sampdata.source_tables,
_cstSourceColumns=sampdata.source_columns,
_cstSourceCodeLists=sampdata.source_codelists,
_cstSourceDocuments=sampdata.source_documents,
_cstSourceValues=sampdata.source_values,
_cstFullModel=N,
_cstLang=en
);The macro is located
in the
global standards library directory/standards/cdisc-definexml-2.0-1.6/macros directory.
Note: The key input (source) files
are the SDTM metadata files (source_study, source_tables, source_columns,
source_codelists, source_values, and source_documents), not the SDTM
domain data sets. The source files to create a Define-XML 2.0 file
have a different structure than the source files to create a CRT-DDS
1.0 file.
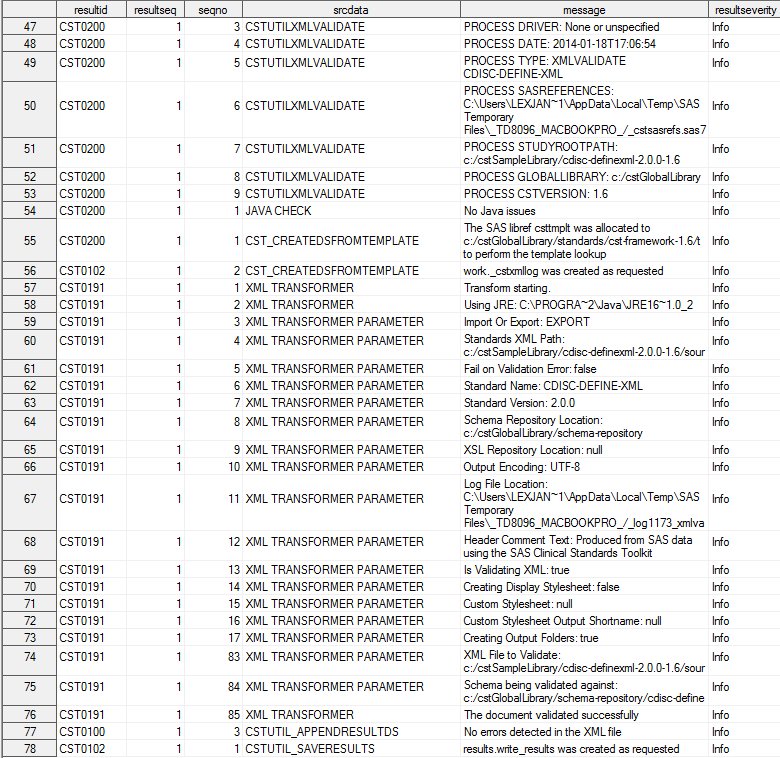
Step 2: Create the define.xml File
At this point, all available
content for the define.xml file has been captured in the SAS representation
(31 data sets) of the CDISC Define-XML 2.0 standard. The SAS Clinical
Standards Toolkit provides a sample driver program, create_definexml.sas.
This program builds and validates the define-sdtm-3.1.2.xml file.
Submit the create_definexml.sas driver program.
In this driver, the
call to the primary task macro requests that the default style sheet
provided by SAS (the source of which is CDISC) be copied to the folder
location containing the generated define.xml file. The macro is located
in the
global standards library directory/standards/cdisc-definexml-2.0-1.6/macros directory.
%define_write(_cstCreateDisplayStyleSheet=1,
_cstHeaderComment=%str(Produced from SAS data using the SAS
Clinical Standards Toolkit &_cstVersion)); Here is a portion of
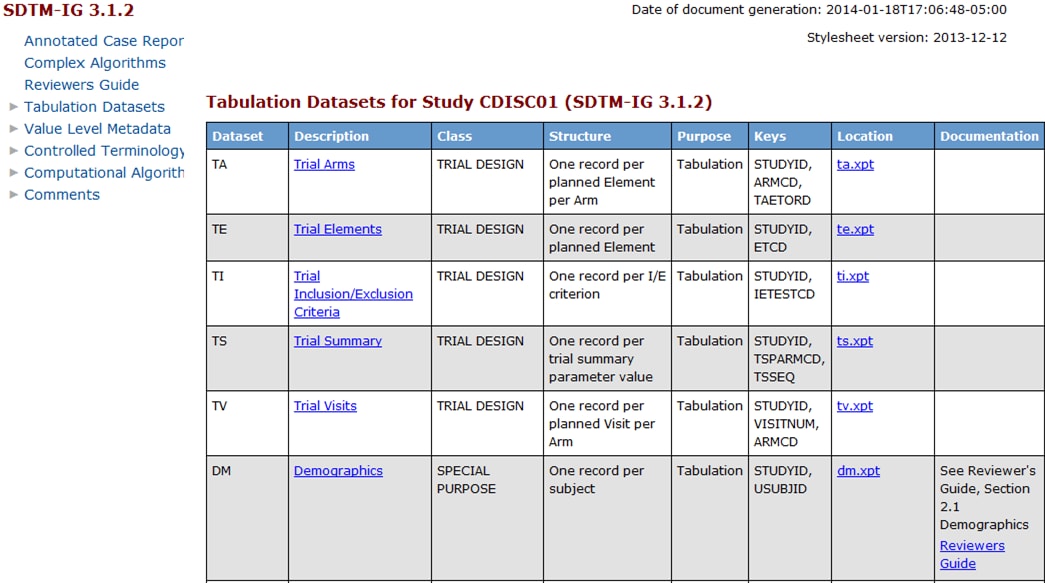
the define-sdtm-3.1.2.xml file as rendered by the default style sheet.
Hyperlinks among tables, columns, codelists, and other file elements
are provided.
Partial Sample define-sdtm-3.1.2.xml File (as Rendered by the
Default Style Sheet)
The final task in the
sample create_definexml.sas driver is to call the cstutilxmlvalidate
macro to perform the schema validation. This involves verifying that
the Define-XML define.xml file is valid structurally and syntactically
according to the XML schema.
Note: The SAS Clinical Standards
Toolkit 1.6
does not contain validation checks to validate the SAS representation
of the Define-XML 2.0 standard. It is expected that version 1.7 of
the SAS Clinical Standards Toolkit will contain validation methodology
that goes beyond XML schema validation.
Copyright © SAS Institute Inc. All rights reserved.