| The SEQTEST Procedure |
Example 78.3 Testing an Effect with Early Stopping to Accept 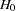
This example demonstrates a two-sided group sequential test that uses an error spending design with early stopping to accept the null hypothesis 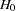 . The example is similar to Example 78.2 but with early stopping to accept
. The example is similar to Example 78.2 but with early stopping to accept 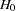 .
.
A study is conducted to examine the effects of Age (years), Weight (kg), RunTime (time in minutes to run 1.5 miles), RunPulse (heart rate while running), and MaxPulse (maximum heart rate recorded while running) on Oxygen (oxygen intake rate, ml per kg body weight per minute). The primary interest is whether oxygen intake rate is associated with weight.
The hypothesis is tested using the following linear model:
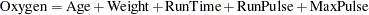 |
The null hypothesis is 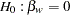 , where
, where 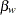 is the regression parameter for the variable Weight. Suppose that
is the regression parameter for the variable Weight. Suppose that 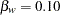 is the reference improvement that should be detected at a
is the reference improvement that should be detected at a 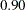 level. Then the maximum information
level. Then the maximum information 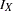 can be derived in the SEQDESIGN procedure.
can be derived in the SEQDESIGN procedure.
Following the derivations in the section "Test for a Parameter in the Regression Model" in the chapter "The SEQDESIGN Procedure," the required sample size can be derived from
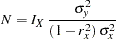 |
where 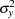 is the variance of the response variable in the regression model,
is the variance of the response variable in the regression model,  is the proportion of variance of Weight explained by other covariates, and
is the proportion of variance of Weight explained by other covariates, and 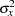 is the variance of Weight.
is the variance of Weight.
Further suppose that from past experience,  ,
, 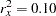 , and
, and  . Then the required sample size can be derived using the SAMPLESIZE statement in the SEQDESIGN procedure.
. Then the required sample size can be derived using the SAMPLESIZE statement in the SEQDESIGN procedure.
The following statements invoke the SEQDESIGN procedure and request a three-stage group sequential design for normally distributed data to test the null hypothesis of a regression parameter 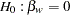 against the alternative
against the alternative 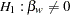 :
:
ods graphics on;
proc seqdesign altref=0.10;
OBFErrorFunction: design method=errfuncgamma
stop=accept
nstages=3
info=cum(2 3 4)
;
samplesize model=reg( variance=5 xvariance=64 xrsquare=0.10);
ods output Boundary=Bnd_Fit;
run;
ods graphics off;
By default, the procedure uses a Type I error probability 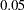 and a Type II error probability
and a Type II error probability 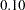 . The ALTREF=0.10 option specifies a power of
. The ALTREF=0.10 option specifies a power of 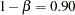 at the alternative hypothesis
at the alternative hypothesis 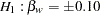 . The INFO=CUM(2 3 4) option specifies that the study perform the first interim analysis with information proportion
. The INFO=CUM(2 3 4) option specifies that the study perform the first interim analysis with information proportion 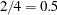 —that is, after half of the total observations are collected.
—that is, after half of the total observations are collected.
The ODS OUTPUT statement with the BOUNDARY=BND_FIT option creates an output data set named BND_FIT which contains the resulting boundary information for the subsequent sequential tests.
The "Design Information" table in Output 78.3.1 displays design specifications and derived statistics. Since the alternative reference is specified, the maximum information is derived.
| Design Information | |
|---|---|
| Statistic Distribution | Normal |
| Boundary Scale | Standardized Z |
| Alternative Hypothesis | Two-Sided |
| Early Stop | Accept Null |
| Method | Error Spending |
| Boundary Key | Both |
| Alternative Reference | 0.1 |
| Number of Stages | 3 |
| Alpha | 0.05 |
| Beta | 0.1 |
| Power | 0.9 |
| Max Information (Percent of Fixed Sample) | 103.9245 |
| Max Information | 1091.972 |
| Null Ref ASN (Percent of Fixed Sample) | 75.00521 |
| Alt Ref ASN (Percent of Fixed Sample) | 101.8099 |
The "Boundary Information" table in Output 78.3.2 displays information level, alternative reference, and boundary values at each stage.
| Boundary Information (Standardized Z Scale) Null Reference = 0 |
|||||||
|---|---|---|---|---|---|---|---|
| _Stage_ | Alternative | Boundary Values | |||||
| Information Level | Reference | Lower | Upper | ||||
| Proportion | Actual | N | Lower | Upper | Beta | Beta | |
| 1 | 0.5000 | 545.9862 | 47.39463 | -2.33663 | 2.33663 | -0.44937 | 0.44937 |
| 2 | 0.7500 | 818.9792 | 71.09195 | -2.86178 | 2.86178 | -1.13583 | 1.13583 |
| 3 | 1.0000 | 1091.972 | 94.78926 | -3.30450 | 3.30450 | -1.91428 | 1.91428 |
With the specified ODS GRAPHICS ON statement, a detailed boundary plot with the rejection and acceptance regions is displayed by default, as shown in Output 78.3.3. The boundary plot also displays the information level and critical value for the corresponding fixed-sample design.
With the MODEL=REG option in the SAMPLESIZE statement, the "Sample Size Summary" table in Output 78.3.4 displays the parameters for the sample size computation.
The "Sample Sizes" table in Output 78.3.5 displays the required sample sizes for the group sequential clinical trial.
Thus, 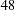 ,
, 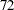 , and
, and 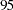 individuals are needed in stages
individuals are needed in stages 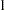 ,
, 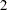 , and
, and 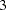 , respectively. Since the sample sizes are derived from estimated values of
, respectively. Since the sample sizes are derived from estimated values of  ,
,  , and
, and 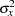 , the actual information levels might not achieve the target information levels. Thus, instead of specifying sample sizes in the protocol, you can specify the maximum information levels. Then if an actual information level is much less than the target level, you can increase the sample sizes for the remaining stages to achieve the desired information levels and power.
, the actual information levels might not achieve the target information levels. Thus, instead of specifying sample sizes in the protocol, you can specify the maximum information levels. Then if an actual information level is much less than the target level, you can increase the sample sizes for the remaining stages to achieve the desired information levels and power.
Suppose that 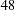 individuals are available at stage
individuals are available at stage 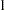 . Output 78.3.6 lists the first
. Output 78.3.6 lists the first 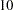 observations of the trial data.
observations of the trial data.
| First 10 Obs in the Trial Data |
| Obs | Oxygen | Age | Weight | RunTime | RunPulse | MaxPulse |
|---|---|---|---|---|---|---|
| 1 | 54.5521 | 44 | 87.7676 | 11.6949 | 178.435 | 181.607 |
| 2 | 52.2821 | 40 | 75.4853 | 9.8872 | 184.433 | 183.667 |
| 3 | 62.1871 | 44 | 89.0638 | 8.7950 | 155.540 | 167.108 |
| 4 | 65.3269 | 42 | 67.7310 | 8.4577 | 162.926 | 173.877 |
| 5 | 59.9809 | 37 | 93.1902 | 9.3228 | 179.033 | 180.144 |
| 6 | 52.5588 | 47 | 75.9044 | 12.0385 | 177.753 | 175.033 |
| 7 | 51.7838 | 40 | 73.5422 | 11.6607 | 175.838 | 178.140 |
| 8 | 57.0024 | 43 | 81.2861 | 11.2219 | 160.963 | 171.770 |
| 9 | 48.0775 | 44 | 85.2290 | 13.1789 | 173.722 | 176.548 |
| 10 | 68.3357 | 38 | 80.2490 | 8.5066 | 171.824 | 184.011 |
The following statements use the REG procedure to estimate the slope 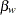 and its associated standard error at stage
and its associated standard error at stage 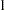 :
:
proc reg data=Fit_1;
model Oxygen=Age Weight RunTime RunPulse MaxPulse;
ods output ParameterEstimates=Parms_Fit1;
run;
The following statements create and display (in Output 78.3.7) the input data set that contains slope 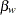 and its associated standard error for the SEQTEST procedure:
and its associated standard error for the SEQTEST procedure:
data Parms_Fit1;
set Parms_Fit1;
if Variable='Weight';
_Scale_='MLE';
_Stage_= 1;
keep _Scale_ _Stage_ Variable Estimate StdErr;
run;
proc print data=Parms_Fit1;
title 'Statistics Computed at Stage 1';
run;
The following statements invoke the SEQTEST procedure to test for early stopping at stage 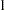 :
:
ods graphics on;
proc seqtest Boundary=Bnd_Fit
Parms(testvar=Weight)=Parms_Fit1
boundaryadj=errfuncgamma
stopprob
order=lr
;
ods output Test=Test_Fit1;
run;
ods graphics off;
The BOUNDARY= option specifies the input data set that provides the boundary information for the trial at stage 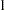 , which was generated in the SEQDESIGN procedure. The PARMS=PARMS_FIT1 option specifies the input data set PARMS_FIT1 that contains the test statistic and its associated standard error at stage
, which was generated in the SEQDESIGN procedure. The PARMS=PARMS_FIT1 option specifies the input data set PARMS_FIT1 that contains the test statistic and its associated standard error at stage 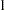 , and the TESTVAR=WEIGHT option identifies the test variable WEIGHT in the data set.
, and the TESTVAR=WEIGHT option identifies the test variable WEIGHT in the data set.
The ORDER=LR option uses the LR ordering to derive the  -value, the unbiased median estimate, and the confidence limits for the regression slope estimate. The boundaries are adjusted using gamma cumulative error spending function, as specified with the BOUNDARYADJ= option.
-value, the unbiased median estimate, and the confidence limits for the regression slope estimate. The boundaries are adjusted using gamma cumulative error spending function, as specified with the BOUNDARYADJ= option.
The ODS OUTPUT statement with the TEST=TEST_FIT1 option creates an output data set named TEST_FIT1 which contains the updated boundary information for the test at stage 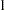 . The data set also provides the boundary information that is needed for the group sequential test at the next stage.
. The data set also provides the boundary information that is needed for the group sequential test at the next stage.
The "Design Information" table in Output 78.3.8 displays the design specifications. By default, the boundary values are modified for the new information levels to maintain the Type I  level. The maximum information remains the same as in the BOUNDARY= data set, but the derived Type II error probability
level. The maximum information remains the same as in the BOUNDARY= data set, but the derived Type II error probability 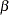 and power
and power 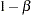 are different because of the new information level.
are different because of the new information level.
| Design Information | |
|---|---|
| BOUNDARY Data Set | WORK.BND_FIT |
| Data Set | WORK.PARMS_FIT1 |
| Statistic Distribution | Normal |
| Boundary Scale | Standardized Z |
| Alternative Hypothesis | Two-Sided |
| Early Stop | Accept Null |
| Number of Stages | 3 |
| Alpha | 0.05 |
| Beta | 0.10003 |
| Power | 0.89997 |
| Max Information (Percent of Fixed Sample) | 103.9351 |
| Max Information | 1091.97232 |
| Null Ref ASN (Percent of Fixed Sample) | 75.11777 |
| Alt Ref ASN (Percent of Fixed Sample) | 101.8186 |
With the STOPPROB option, the "Expected Cumulative Stopping Probabilities" table in Output 78.3.9 displays the expected stopping stage and the cumulative stopping probability of accepting the null hypothesis at each stage under various hypothetical references 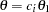 , where
, where 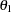 is the alternative reference and
is the alternative reference and  by default. You can specify other values for
by default. You can specify other values for 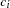 with the CREF= option.
with the CREF= option.
| Expected Cumulative Stopping Probabilities Reference = CRef * (Alt Reference) |
|||||
|---|---|---|---|---|---|
| CRef | Expected Stopping Stage |
Source | Stopping Probabilities | ||
| Stage_1 | Stage_2 | Stage_3 | |||
| 0.0000 | 1.905 | Accept Null | 0.33294 | 0.76168 | 0.95000 |
| 0.5000 | 2.417 | Accept Null | 0.17674 | 0.40627 | 0.62824 |
| 1.0000 | 2.920 | Accept Null | 0.02635 | 0.05405 | 0.10003 |
| 1.5000 | 2.997 | Accept Null | 0.00109 | 0.00165 | 0.00242 |
The "Test Information" table in Output 78.3.10 displays the boundary values for the test statistic with the default standardized  scale. The information level at stage
scale. The information level at stage 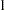 is derived from the standard error
is derived from the standard error  in the PARMS= data set,
in the PARMS= data set,
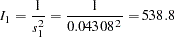 |
| Test Information (Standardized Z Scale) Null Reference = 0 |
||||||||
|---|---|---|---|---|---|---|---|---|
| _Stage_ | Alternative | Boundary Values | Test | |||||
| Information Level | Reference | Lower | Upper | Weight | ||||
| Proportion | Actual | Lower | Upper | Beta | Beta | Estimate | Action | |
| 1 | 0.4934 | 538.7887 | -2.32118 | 2.32118 | -0.43019 | 0.43019 | 1.08174 | Continue |
| 2 | 0.7467 | 815.3805 | -2.85549 | 2.85549 | -1.12435 | 1.12435 | . | |
| 3 | 1.0000 | 1091.972 | -3.30450 | 3.30450 | -1.91475 | 1.91475 | . | |
The information levels at stages 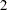 and
and 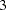 are derived proportionally from the corresponding information levels in the BOUNDARY= data set. See the section Boundary Adjustments for Information Levels for details about how these information levels are computed.
are derived proportionally from the corresponding information levels in the BOUNDARY= data set. See the section Boundary Adjustments for Information Levels for details about how these information levels are computed.
At stage 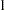 , the standardized
, the standardized  statistic
statistic 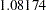 is greater than the upper
is greater than the upper 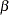 boundary
boundary 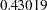 , so the trial continues to the next stage.
, so the trial continues to the next stage.
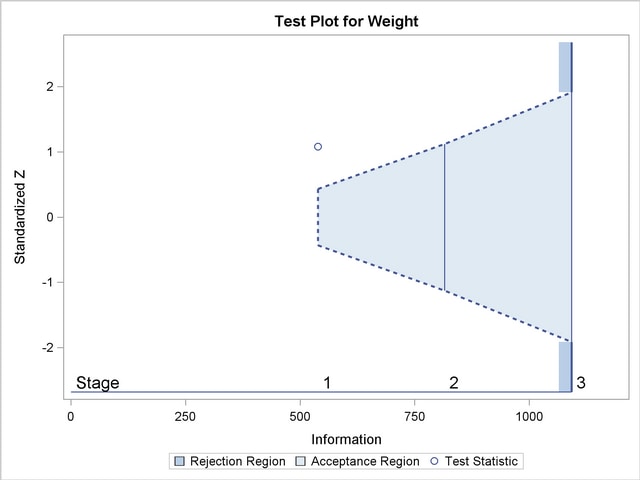
With the specified ODS GRAPHICS ON statement, a boundary plot with test statistics is displayed by default, as shown in Output 78.3.11. As expected, the test statistic is in the continuation region.
The following statements use the REG procedure to estimate the slope 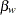 and its associated standard error at stage
and its associated standard error at stage 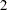 :
:
proc reg data=Fit_2;
model Oxygen=Age Weight RunTime RunPulse MaxPulse;
ods output ParameterEstimates=Parms_Fit2;
run;
Note that the data set Fit_2 contains both the data from stage 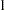 and the data from stage
and the data from stage 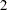 ,
,
The following statements create and display (in Output 78.3.12) the input data set that contains slope 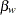 and its associated standard error at stage
and its associated standard error at stage 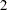 for the SEQTEST procedure:
for the SEQTEST procedure:
data Parms_Fit2;
set Parms_Fit2;
if Variable='Weight';
_Scale_='MLE';
_Stage_= 2;
keep _Scale_ _Stage_ Variable Estimate StdErr;
run;
proc print data=Parms_Fit2;
title 'Statistics Computed at Stage 2';
run;
The following statements invoke the SEQTEST procedure to test for early stopping at stage 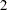 :
:
ods graphics on;
proc seqtest Boundary=Test_Fit1
Parms(testvar=Weight)=Parms_Fit2
boundaryadj=errfuncgamma
order=lr
pss
plots=(asn power)
;
ods output Test=Test_Fit2;
run;
ods graphics off;
The BOUNDARY= option specifies the input data set that provides the boundary information for the trial at stage 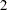 , which was generated by the SEQTEST procedure at the previous stage. The PARMS= option specifies the input data set that contains the test statistic and its associated standard error at stage
, which was generated by the SEQTEST procedure at the previous stage. The PARMS= option specifies the input data set that contains the test statistic and its associated standard error at stage 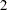 , and the TESTVAR= option identifies the test variable in the data set.
, and the TESTVAR= option identifies the test variable in the data set.
Since the data set PARMS_FIT2 does not contain the test information at stage 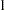 , the information level at stage
, the information level at stage 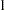 in the TEST_FIT1 data set is used to generate boundary values for the test.
in the TEST_FIT1 data set is used to generate boundary values for the test.
The ORDER=LR option uses the LR ordering to derive the  -value, unbiased median estimate, and confidence limits for the regression slope estimate.
-value, unbiased median estimate, and confidence limits for the regression slope estimate.
The ODS OUTPUT statement with the TEST=TEST_FIT2 option creates an output data set named TEST_FIT2 which contains the updated boundary information for the test at stage 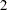 . The data set also provides the boundary information that is needed for the group sequential test at the next stage.
. The data set also provides the boundary information that is needed for the group sequential test at the next stage.
The "Design Information" table in Output 78.3.13 displays design specifications. By default, the boundary values are modified for the new information levels to maintain the Type I  level.
level.
| Design Information | |
|---|---|
| BOUNDARY Data Set | WORK.TEST_FIT1 |
| Data Set | WORK.PARMS_FIT2 |
| Statistic Distribution | Normal |
| Boundary Scale | Standardized Z |
| Alternative Hypothesis | Two-Sided |
| Early Stop | Accept Null |
| Number of Stages | 3 |
| Alpha | 0.05 |
| Beta | 0.10009 |
| Power | 0.89991 |
| Max Information (Percent of Fixed Sample) | 103.9552 |
| Max Information | 1091.97232 |
| Null Ref ASN (Percent of Fixed Sample) | 75.18481 |
| Alt Ref ASN (Percent of Fixed Sample) | 101.8341 |
The derived Type II error probability 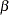 and power
and power 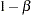 are different because of the new information levels.
are different because of the new information levels.
With the PSS option, the "Power and Expected Sample Sizes" table in Output 78.3.14 displays powers and expected mean sample sizes under various hypothetical references 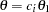 , where
, where 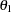 is the alternative reference and
is the alternative reference and  are the default values in the CREF= option.
are the default values in the CREF= option.
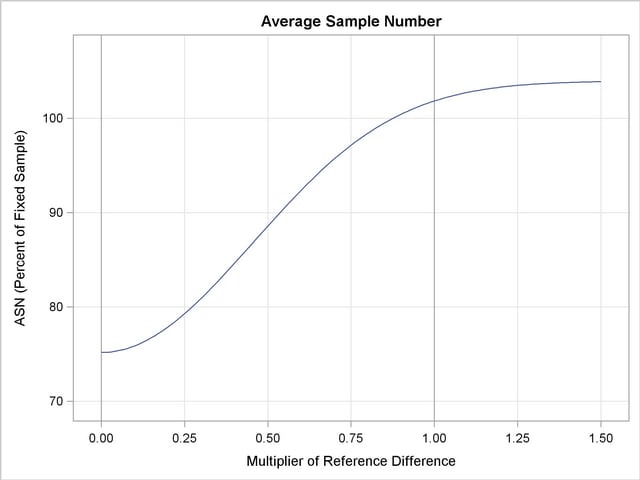
With the PLOTS=ASN option, the procedure displays a plot of expected sample sizes under various hypothetical references, as shown in Output 78.3.15. By default, expected sample sizes under the hypotheses 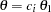 ,
,  , are displayed, where
, are displayed, where 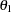 is the alternative reference.
is the alternative reference.
With the PLOTS=POWER option, the procedure displays a plot of the power curves under various hypothetical references for all designs simultaneously, as shown in Output 78.3.16. By default, powers under hypothetical references 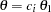 are displayed, where
are displayed, where  by default. You can specify
by default. You can specify 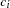 values with the CREF= option. The
values with the CREF= option. The 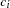 values are displayed on the horizontal axis.
values are displayed on the horizontal axis.
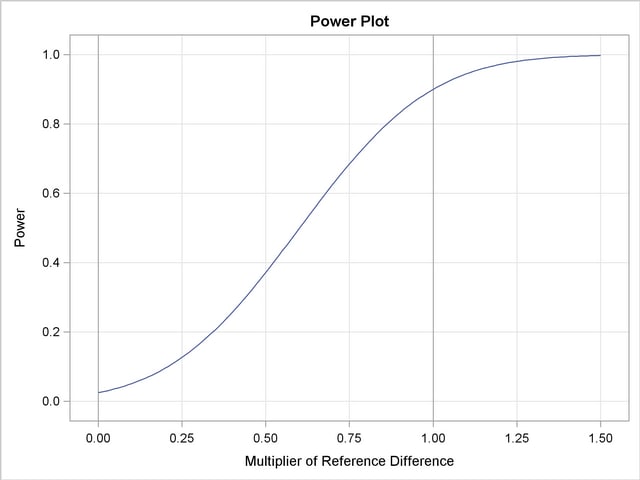
Under the null hypothesis, 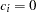 , the power is
, the power is  , which is the upper Type I error probability. Under the alternative hypothesis,
, which is the upper Type I error probability. Under the alternative hypothesis,  , the power is
, the power is 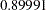 , which is one minus the Type II error probability, as displayed in the "Design Information" table in Output 78.3.13.
, which is one minus the Type II error probability, as displayed in the "Design Information" table in Output 78.3.13.
The "Test Information" table in Output 78.3.17 displays the boundary values for the test statistic with the default standardized  scale. At stage
scale. At stage 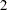 , the standardized slope estimate
, the standardized slope estimate 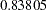 is between the lower and upper
is between the lower and upper  boundary values. The trial stops to accept the null hypothesis that the variable Weight has no effect on the oxygen intake rate after adjusting for other covariates.
boundary values. The trial stops to accept the null hypothesis that the variable Weight has no effect on the oxygen intake rate after adjusting for other covariates.
| Test Information (Standardized Z Scale) Null Reference = 0 |
||||||||
|---|---|---|---|---|---|---|---|---|
| _Stage_ | Alternative | Boundary Values | Test | |||||
| Information Level | Reference | Lower | Upper | Weight | ||||
| Proportion | Actual | Lower | Upper | Beta | Beta | Estimate | Action | |
| 1 | 0.4934 | 538.7887 | -2.32118 | 2.32118 | -0.43019 | 0.43019 | 1.08174 | Continue |
| 2 | 0.7517 | 820.8509 | -2.86505 | 2.86505 | -1.14237 | 1.14237 | 0.83805 | Accept Null |
| 3 | 1.0000 | 1091.972 | -3.30450 | 3.30450 | -1.91409 | 1.91409 | . | |
Since the data set PARMS_FIT2 contains the test information only at stage 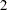 , the information level at stage
, the information level at stage  in the TEST_FIT1 data set is used to generate boundary values for the test.
in the TEST_FIT1 data set is used to generate boundary values for the test.
With the specified ODS GRAPHICS ON statement, a boundary plot with test statistics is displayed by default, as shown in Output 78.3.18. As expected, the test statistic is in the acceptance region between the lower and upper  boundaries at the final stage.
boundaries at the final stage.
After a trial is stopped, the "Parameter Estimates" table in Output 78.3.19 displays the stopping stage, parameter estimate, unbiased median estimate, confidence limits, and the  -value under the null hypothesis
-value under the null hypothesis  . As expected, the
. As expected, the  -value
-value 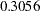 is not significant at the
is not significant at the 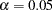 level, and the confidence interval does contain the value zero. The
level, and the confidence interval does contain the value zero. The  -value, unbiased median estimate, and confidence limits depend on the ordering of the sample space
-value, unbiased median estimate, and confidence limits depend on the ordering of the sample space 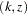 , where
, where 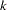 is the stage number and
is the stage number and  is the standardized
is the standardized  statistic. With the specified LR ordering, the
statistic. With the specified LR ordering, the  -values are computed with the ordering
-values are computed with the ordering 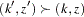 if
if 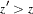 . See the section Available Sample Space Orderings in a Sequential Test for a detailed description of the LR ordering.
. See the section Available Sample Space Orderings in a Sequential Test for a detailed description of the LR ordering.
Copyright © 2009 by SAS Institute Inc., Cary, NC, USA. All rights reserved.