Building a Validation Process
Overview
Building a SAS Clinical
Standards Toolkit validation process is similar to building any SAS
Clinical Standards Toolkit process. The differences are the validation
process inputs and outputs, as defined in the SASReferences data set,
can differ, a standard-specific validate macro is called, and process
output can include an optional Metrics data set.
This table shows the
standard-specific validation macros for all SAS Clinical Standards
Toolkit standards that support validation.
SASReferences Customizations
A SAS Clinical Standards
Toolkit validation process requires that you specify a reference standard
with which the source data and metadata can be compared. These three
records, specific to the standard and standardversion of interest,
should be included in the SASReferences data set:
Defining the Reference Standard in the SASReferences Data Set
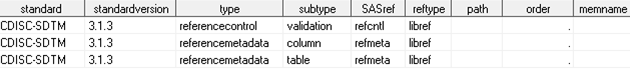
The empty path field
signals that the path and memname information should be derived from
the StandardSASReferences data set associated with the standard and
standardversion. Including the referencecontrol and referencemetadata
records is unique to validation process in the SAS Clinical Standards
Toolkit.
-
The Validation.Properties file sets process global macro variables specific to validation, such as metrics. For a complete discussion of these properties, see Validation.Properties. For information about the derived global macro variables, see Global Macro Variables. The Validation.Properties file is a required file to support the SAS Clinical Standards Toolkit validation.
-
The Metrics data set provides a summary of the validation process, including error counts, processing time, and denominators for specific checks. For a complete discussion of validation metrics, see Validation Metrics and Validation Results and Metrics. For information about the global macro variables that govern metrics output, see Global Macro Variables. The Metrics data set is typically output to the same location as the validation Results data set. This location is common to all SAS Clinical Standards Toolkit processes.
-
The location of any libraries containing controlled terminology, format catalogs, and coding dictionary data sets.The type=fmtsearch records enable you to specify multiple format catalogs (for example, company-wide, compound, group-level, and study-level). Order in the format search path is set by the order field. The type=referencecterm record enables you to specify one or more lookup data sets (such as dictionary lookups like LOINC and MedDRA). These lookup data sets do not need to conform to a specific structure, and they do not need to be in a structure that can be read into a SAS format. Customized code (typically in the Validation Master codelogic field) is required to join domain data with each associated lookup data set.
Validation Control: Specification of Run-Time Checks
Each SAS Clinical Standards
Toolkit validation process requires you to specify the validation
checks to be run. This is accomplished by cloning, subsetting, or
building a set of validation checks based on the Validation Master
data set. (See Validation Check Metadata: Validation Master.) The SAS Clinical Standards Toolkit assumes that each Validation
Control data set is structurally equivalent to the Validation Master
data set.
As a required input
to a validation process, the Validation Control data set must be referenced
in the run-time SASReferences file. (See Defining the Run-Time Validation Control Location in the SASReferences Data Set.)
The &studyRootPath
value is assumed to have been set to
sample study library directory/cdisc-sdtm-3.1.3/sascstdemodata.
The Validation Master
data set (illustrated
in Defining the Reference Standard in the SASReferences Data Set and
in this display) serves as the source for Validation Control content.
Note that in this display, the path and memname information
have been derived from the StandardSASReferences data set and points
to the global standards library.
Defining Validation Control Data Set Location

This table provides
examples of how to create a Validation Control data set from the Validation
Master data set. The sample code is written assuming that the code
will be submitted in a context where libraries have been allocated
and the format search and autocall paths have been set.
Generally, the SAS Clinical
Standards Toolkit processes validation checks in the order in which
they appear in the Validation Control data set. Each validation process
honors the default validation property _cstCheckSortOrder. If this
property is not set, then the data set order is assumed. As a part
of the Validation Control derivation, checks can be sorted in any
user-defined order. Or, _cstCheckSortOrder can be set to sort the
Validation Control data set at run time by any fields in that data
set.
Setting Properties for the Validation Process
Across all standards,
the set of properties that are available for a validation process
is extensive. (For more
information about the full set of validation properties, see Global Macro Variables.) However, only a few properties are modified on a regular
basis. These include:
These changes should
be made before the process setup begins (as changes to the properties
file), or after the process setup ends (as a series of %let statements
in the code stream).
Tip
Best Practice Recommendation:
Centralizing property changes in properties files, rather than distributing
them in code segments, offers advantages for debugging and documenting
processes. Properties are translated to global macro variables by
calls to the cst_setstandardproperties or cst_setproperties framework
utility macros during process setup. They are reported in the SAS
log, and are generally documented in the process SASReferences file.
Copyright © SAS Institute Inc. All rights reserved.



