Metadata Requirements
Overview
As noted in Supported Standards, a standard consists of properties, messages, and metadata
files that collectively represent the standard in the SAS Clinical
Standards Toolkit. Each SAS Clinical Standards Toolkit registered
standard can support validation if the standards.supportsvalidation
flag is set to Y. This setting indicates that the required set of
validation files defining the standard exist. By default, the set
of validation files that supports the standards that are supplied
by SAS is in the cstGlobalLibrary folder hierarchy.
For example, validation
files that define the CDISC SDTM 3.1.3 standard are in this folder
hierarchy:
The following sections
describe each metadata type used by typical validation processes. For information
about metadata files that are common to all SAS Clinical Standards
Toolkit processes, see Metadata File Descriptions. Metadata characteristics specific
to compliance assessments are described in the sections in this chapter.
Reference Metadata
For CDISC standards,
reference metadata about data sets is defined in a reference_tables
data set, and metadata about columns is defined in a reference_columns
data set. An example
of a reference_tables record is provided in reference_tables Data Set and an example
of a reference_columns record is provided in reference_columns Data Set.
As noted in Supported Standards, each standard
that is supplied by SAS provides a SAS interpretation of the published
source guidelines or specification of that standard. Each standard is designed to serve as a representative
model or template of the source specification. Each model or template
can be modified to establish your own gold standard.
|
The path to the SAS
transport file. This path can be specified as a relative path. The
value can be used when creating define.xml to populate the value for
the def:leaf xlink:href link to the domain file. The value should
be the pathname and filename of the SAS transport file relative to
the location of define.xml file. This column is optional and not relevant
for all standards.
|
||
|
The title of the SAS
transport file. The value can be used when creating a define.xml file
to populate the value for the def:leaf def:title value. It can provide
a meaningful description, label, or location of the domain leaf (for
example, crt/datasets/Protocol 1234/AE.xpt). This column is optional
and not relevant for all standards.
|
||
|
This value captures
the standard name. This value must match the name of a registered
standard in the SAS Clinical Standards Toolkit framework. For a discussion
of registered standards, see Framework. This value must match the standard
field in the SASReferences data set. Examples are CDISC SDTM and CDISC
CRT-DDS. This column is required.
|
||
|
A SAS format name that
is used to assess conformance to controlled terminology. This value
does not have a $ prefix for character formats and does not have the
trailing period. This value is also the codelist name in the define.xml
file. The SAS format name must be in the format search path for successful
column-value validation. This record is optional and not relevant
for all standards.
|
||
|
This value captures
the standard name. This value must match the name of a registered
standard in the SAS Clinical Standards Toolkit framework. For a discussion
of registered standards, see Framework. This value must match the standard
field in the SASReferences data set. Examples are CDISC SDTM and CDISC
CRT-DDS. This column is required.
|
||
The standard reference
metadata provided by SAS is in the SAS Clinical Standards Toolkit
global standards library. By default, this library is here:
This global standards
library metadata folder can contain other standard-specific metadata.
For example, CDISC SDTM includes class_tables and class_columns data
sets. These data sets have more generic metadata than specific domain
instances like DM or AE, and they are most useful when deriving new,
custom domains. For example, if a new CDISC SDTM events domain is
required, you can initialize table metadata based on the EVENTS record
in class_tables data set, and can initialize column metadata based
on the EVENTS, IDENTIFIERS, and TIMING records in the class_columns
data set.
Source Metadata
The SAS Clinical Standards
Toolkit validation processes require source metadata that describes
source (study) domains and columns. This is the study data that is
to be validated. The SAS Clinical Standards Toolkit assumes that the
reference metadata (that is, reference_tables and reference_columns)
for a standard serves as a model or template for the source metadata
(that is, source_tables and source_columns). It is recommended that
these two sets of metadata be structurally equivalent. However, additional
metadata attributes might exist if they are used for other purposes
or for custom extensions to the SAS Clinical Standards Toolkit.
The SAS Clinical Standards
Toolkit assumes that source_tables and source_columns data sets accurately
reflect and are consistent with the source data that they describe.
Although some standard-specific validation checks might look for discrepancies
and report them in detail, failure to accurately reflect and be consistent
with the source data can lead to errors in the SAS Clinical Standards
Toolkit validation process. It can even halt the execution of the
process.
Validation Check Metadata: Validation Master
The Validation Master
data set contains all validation checks defined for a standard. By
default, this data set is deployed to this directory in each supported
standard:
This table lists the
columns in the Validation Master data set. These columns are described
and examples are reviewed in the following sections.
|
Validation check ID.
The SAS Clinical Standards Toolkit has adopted a naming convention
matching each standard to be validated. The checkid values are prefixed
with an up to 4-character prefix (CDISC examples: ODM, SDTM, ADAM,
and CRT). By convention, the prefix matches the mnemonic field in
the Standards data set in
global standards library directory/metadata.
This prefix is followed by a 4-digit numeric that is unique within
the standard (for example, SDTM1234). You can use any naming convention
limited to eight characters. By default, the checkid column is the
first (primary) sort field in the Validation Master data set provided
by SAS. Sorting by checkid is not required. This column is required.
|
||
|
This value captures
the standard name. This value must match the name of a registered
standard in the SAS Clinical Standards Toolkit framework. For a discussion
of registered standards, see Framework. This value must match the standard
field in the SASReferences data set. Examples are CDISC SDTM and CDISC
CRT-DDS. This column is required.
|
||
|
This value captures
a specific version of a standard. This value must match one of the
standard versions associated with a registered standard. This value
must match the standardversion field in the SASReferences data set.
The only exception to this rule is that *** can be used to signify
that the check applies to all supported versions of the standard.
For example, 3.1.1, 1.0, ***. If a subsequent version of the standard
is released, then *** would be applicable if the check is valid for
the new version. This column is required.
|
||
|
A string that identifies
the source of the check. CDISC examples include Janus, JanusFR (FAIL-REJECT),
SAS, WebSDM, and OpenCDISC. This field can contain any user-defined
value. A primary use of this field is to subset the full set of checks
in the run-time Validation Control data set. This column is required.
|
||
|
The value specifies
the domains to be validated by the check. The domains must exist in
either or both of the reference metadata or source metadata. The value
can be in the form:
_ALL_-DM-DS: Multiple
domains that exclude one or more specific domains that are delimited
with a -.
CLASS:EVENTS: All domains
capturing event results. (This syntax specifies to use table metadata
column CLASS for EVENTS as the value-similar syntax for all other
fields and values.)
|
||
|
The value specifies
one or more space-delimited columns identified for inclusion or exclusion
in the specified check. The value can be in the form:
|
||
|
Check-specific code
segment that is inserted into the check macro defined in codesource
and consistent with codetype. The codelogic value enables check-level
customization and allows the reuse of more general check macros. The
field length of $2000 limits the code to short code segments, although
referencing another macro or using %include expands this capability.
The codelogic value can use global and local macro variables (for
example, variables provided as macro input parameters and variables
set within the calling code). Examples include:
|
||
|
This value defines whether
to use codelogic and what type of codelogic can be used in the validation
code. Values include:
3: Calls a SAS macro
or %include that can contain only DATA step statement level code.
(For example, codetype=1.)
|
||
|
This value defines the
type of information to use for value comparison to some standard.
Values include:
Metadata: Use the SAS
Clinical Standards Toolkit metadata. Specifically, use the value of
the column metadata field xmlcodelist to identify the codelist (rendered
as a SAS format).
Dataset: Use a reference
SAS data set (for example, medDRA). There are no SAS Clinical Standards
Toolkit requirements for the structure and content of the reference
SAS data set.
|
||
|
If lookuptype is metadata,
then lookupsource should be blank. The code gets the value from the
source_columns.xmlcodelist field.
If lookuptype is format,
then lookupsource should be the SAS format and must be in the format
search path if it is specified. This value should generally match
any value in source_columns.xmlcodelist for the columns specified
in columnscope. This field allows a run-time validation check against
another format.
If lookuptype is dataset,
then lookupsource should be the name of a SAS data set. This value
is specified as the data set name (for example, meddra) or libref.dataset.
If a value is provided without a libref, then the SAS Clinical Standards
Toolkit looks for any SASReferences type=referencecterm records for
the sasref value.
|
||
|
This value includes
columns not included in columnscope for code-processing purposes and
to help resolve errors. If this value is specified, then it should
be a space-delimited list of columns in the domains specified in the
tablescope field. The values of these columns can be reported in the
Results data set. This column is optional.
|
||
|
This value provides
a unique ID for the check. It ensures uniqueness in the data set and
in the SAS Clinical Standards Toolkit. This value allows any provided
or derived check to be uniquely identifiable over time. An example
is SDTM000100CST120SDTM3112009-05-12T12:00:00CDI.
|
||
The content of the Validation
Master data set is based on a combination of compliance requirements
and the SAS representation of the standard.
This table describes
a sample Validation Master data set record for the CDISC SDTM 3.1.2
standard.
The Validation Master
data set contains all validation checks for a standard, whereas the
Validation Control data set is the run-time equivalent and contains
just the validation checks to be run in a validation process. The
Validation Control data set is structurally equivalent to the Validation
Master data set. For additional
information about how the validation check metadata in the Validation
Control data set is used in the SAS Clinical Standards Toolkit validation
processes, see Special Topic: How the SAS Clinical Standards Toolkit Interprets Validation Check Metadata.
Supplemental Validation Check Metadata: Validation Standard References
The validation standard
references data set contains additional information about each of
the checks in the Validation Master data set. This data set is used
in the validation metadata reporting process to provide additional
information to you about the origin of the check. It also provides
any supporting documentation about the check. By default, this data
set is deployed to this directory in each supported standard:
|
The validation check
ID, as specified in the Validation Master data set. (See Column Descriptions of the Validation Master Data Set.)
|
||
The content of the Validation_StdRef
data set is based on information from any source that supports the
check.
This table describes
information about a specific check in the Validation_StdRef data set
(record 1) for the CDISC SDTM 3.1.2 standard.
Supplemental Validation Check Metadata: CDISC SDTM Domains by Check
The SAS Clinical Standards
Toolkit validation metadata, as specified in the Validation Master
data set, uses the tablescope and columnscope columns to define the
scope of the check. The scope being what domains (tables) and what
columns will be validated when the check is run. The SAS Clinical
Standards Toolkit uses a shorthand syntax in these columns that is
interpreted by the SAS Clinical Standards Toolkit framework macros
to build a list of target tables and columns. For more information,
see Special Topic: How the SAS Clinical Standards Toolkit Interprets Validation Check Metadata. The Validation_DomainsByCheck data set is supplied in
global standards library directory/standards/cdisc-sdtm-3.1.x/validation/control.
It contains records for each domain that is to-be-validated by each
check in the Validation Master data set. This data set is used by
reporting tools that are provided with the SAS Clinical Standards
Toolkit to report domain-specific errors. For more information,
see Reporting. It is also available to other programs and applications
that might need to subset checks that are applicable to specific domains.
The SDTM version of
the Validation_DomainsByCheck data set that is supplied by SAS is
built from the version of the Validation Master data set that is also
supplied by SAS. If the tableScope and columnScope columns are modified,
then the Validation_DomainsByCheck data set must also be modified
or rebuilt.
|
The validation check
ID, as specified in the Validation Master data set. (See Column Descriptions of the Validation Master Data Set.)
|
||
For CDISC SDTM 3.1.2
validation check SDTM0207, the Validation_DomainsByCheck data set
contains records for 14 domains. These 14 domains are DA, EG, FA,
IE, LB, MB, MS, PC, PE, PP, QS, SV, TV, and VS. The target domains
and columns for check SDTM0207 are defined as tableScope=_ALL_ and
columnScope=VISITNUM. This means there are 14 domains in the sample
study metadata provided for CDISC SDTM 3.1.2 that contain the column
VISITNUM.
Supplemental Validation Check Metadata: CDISC ADaM Class by Check
For CDISC ADaM, the
supplemental data set is called Validation_ClassByCheck. It is located
at:
global standards library directory/standards/cdisc-adam-2.1-1.5/validation/control.
This data set
is patterned after the data set that is described in Column Descriptions of the Validation_DomainsByCheck Data Set. However, the column class ($40, Observation Class within
Standard) has been added. This addition accommodates the different
way that the ADaM reference standard is defined. For example, the
reference_tables data set, located in
/standards/cdisc-adam-2.1-1.5/metadata,
includes a BDS record that serves as a class template for all specific
implementations of BDS that are required for a study. The SAS Clinical
Standards Toolkit does not know each of the specific analysis data
sets, so the Validation_ClassByCheck data set includes records by
class, not by domain, for each check in the ADaM Validation Master
data set.
Validation.Properties
Properties specific
to validation processes are provided with the SAS Clinical Standards
Toolkit. These properties enable you to specify how validation checks
are to be processed and whether metrics are to be reported.
As with all SAS Clinical
Standards Toolkit properties files, a call to the %cst_setproperties
macro is required to translate the properties into SAS global macro
variables. This call can be explicitly made as a driver module setup
task, or it can be made by including the Validation.Properties file
as a record in the SASReferences data set. For all standards that
support validation, the Validation.Properties file is required, even
if no metrics are wanted because the SAS Clinical Standards Toolkit
validation process does expect, and will use, the metrics global macro
variables.
This table describes
the properties in the Validation.Properties file.
Messages
Each SAS Clinical Standards
Toolkit registered standard that supports validation has a Validation
Master data set, and an associated Messages data set. The Validation
Master data set provides the super-set of checks defined for that
standard. The Messages data set provides messages to be generated
during the execution of each validation process. A distinct Messages
data set record is expected for each set of checkid and checksource
values in the Validation Master data set. Messages can be parameterized
and internationalized.
By default, the standard-specific
Messages data set is deployed to this directory in each supported
standard:
All Messages data sets
in the SAS Clinical Standards Toolkit should have the same structure. The structure
is defined in Metadata File Descriptions.
During a process, the
SAS Clinical Standards Toolkit appends any standard-specific messages
that are required by the process to any generic SAS Clinical Standards
Toolkit framework messages that are available to all processes. This
appended Messages data set follows the naming convention that is defined
within the global macro variable _cstMessages.
Validation Metrics
Generating the SAS Clinical
Standards Toolkit validation metrics provides a meaningful denominator
for most validation checks. This enables you to more accurately assess
the relative scope of errors that are detected. Generally, the calculated
denominator is a count of the number of records processed in a domain.
This code segment, which
is extracted from a validation check macro, shows a typical calculation
of the number of records in a domain. It also shows the macro call
to add the count to the Validation Metrics data set:
data _null_;
if 0 then set &_cstDSName nobs=_numobs;
call symputx('_cstMetricsCntNumRecs',_numobs);
stop;
run;
* Write applicable metrics *;
%if &_cstMetrics %then %do;
%if &_cstMetricsNumRecs %then
%cstutil_writemetric(
_cstMetricParameter=# of records tested,
_cstResultID=&_cstCheckID,
_cstResultSeqParm=&_cstResultSeq,
_cstMetricCnt=&_cstMetricsCntNumRecs,
_cstSrcDataParm=&_cstDSname
);
%end;
Because a check can
evaluate multiple columns in a domain, the count will be greater.
In addition, a metadata-level check that does not access the domain
data directly might report the number of metadata records instead.
Metrics processing is
enabled based on settings in the Validation.Properties file. See Properties in the Validation.Properties File.
This table provides
a description of the Validation Metrics data set, including the meaning
of each field.
This display illustrates
Validation Metrics output from a SAS Clinical Standards Toolkit validation
process running CDISC SDTM 3.1.1 validation. The Validation Control
data set contains three records: two SDTM0451 checks and one SDTM0623
check.
Sample Validation Metrics Data Set
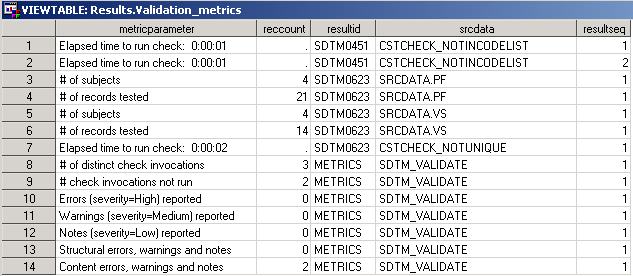
Lines 1 through 2 document
that the SDTM0451 check was invoked twice. The missing recount value
and the absence of other metrics indicate that the two check invocations
failed. This should be reported in the Results data set.
Lines 3 through 7 provide
metrics information about the SDTM0623 check. SDTM0623 checks that
multiple standard units do not exist for any test in the findings
domains. The SDTM0623 check was run on two domains using the cstcheck_notunique
check macro. The number of subjects and records tested, and the elapsed
time to run the check are reported.
Lines 8 through 14 are
summary metrics reported at the end of the SDTM validation process
in the sdtm_validate macro. There are no errors. It is noted that
two checks could not be run (lines 9 and 14).
For more information
about the Validation Metrics data set, see Column Descriptions of the Validation Metrics Data Set.
Copyright © SAS Institute Inc. All rights reserved.