Special Topic: A Round Trip Exercise Involving the CDISC CRT-DDS Standard: Importing and Exporting the define.xml File
Overview
In general, when representing
an XML-based standard in SAS, an XML element is mapped to a SAS data
set and its associated attributes are mapped to the columns of the
SAS data set. When the SAS Clinical Standards Toolkit creates a CDISC
CRT-DDS 1.0 XML file, it converts the information from a SAS data
set representation of the CRT-DDS model into XML. For CDISC CRT-DDS
1.0, this means that 39 data sets (such as ItemDefs) containing 176
columns are the source for creating the define.xml element and attribute
structure. The SAS representation of the CRT-DDS standard can be derived
in part from other standards (such as CDISC SDTM) and can include
supporting metadata from other sources.
The first step in creating
a define.xml file with the SAS Clinical Standards Toolkit is populating
the SAS data set representation of the CRT-DDS model from the SDTM
domain metadata (source_tables and source_columns data sets) and the
study metadata (source_study data set) by running the crtdds_sdtmtodefine
macro. Depending on the completeness of this source data, the crtdds_sdtmtodefine
macro can (partially) populate these 12 of the 39 CRT-DDS SAS tables:
The remainder of the
tables will not be automatically populated by the SAS Clinical Standards
Toolkit.
Sample Driver Program: import_sascrtdds_fromxml_export_toxml.sas
Overview
The SAS Clinical Standards
Toolkit provides a driver program, import_sascrtdds_fromxml_export_toxml.sas,
to demonstrate import and export of extensive CRT-DDS metadata.
This program provides
the same process setup function supported in most SAS Clinical Standards
Toolkit driver modules, using a SASReferences data set that defines
process inputs and outputs, and allocating all SAS librefs and filerefs.
In this sample driver program, the SASReferences data sets are not
created in the program, but rather read from a permanent SAS data
set.
The SASReferences Data Set
As a part of
each SAS Clinical Standards Toolkit process setup, a valid SASReferences
data set is required. It references the input files that are needed,
the librefs and filenames to use, and the names and locations of data
sets to be created by the process. It can be modified to point to
study-specific files. For
an explanation of the SASReferences data set, see SASReferences File.
Key Components of the SASReferences Data Set import_sasreference and Key Components of the SASReferences Data Set export_sasreferences list the files
and data sets that are key components in the SASReference files that
are used in the sample driver program import_sascrtdds_fromxml_export_toxml.sas. In this driver program, these values are set for &studyRootPath
and &studyOutputPath:
&studyRootPath=!sasroot /../../SASClinicalStandardsToolkitCRTDDS10/&_cstVersion/sample/cdisc-crtdds-1.0 &studyOutputPath=!sasroot /../../SASClinicalStandardsToolkitCRTDDS10/&_cstVersion/sample/cdisc-crtdds-1.0 Process Outputs
When running the sample
driver program interactively, you can verify in the Work library the
SAS representation of the CRT-DDS model contains observations for
these CRT-DDS data sets.
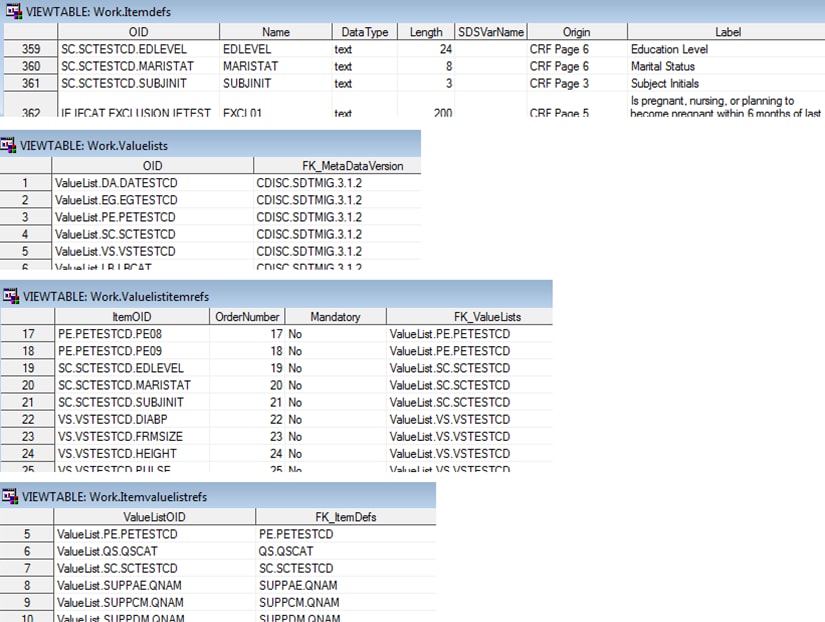
This example shows how
the XML code from the CRT-DDS file define_import.xml has been imported
in to four SAS CRT-DDS data sets (itemdefs, valuelists, valuelistitemrefs,
and itemvaluelistrefs) in the Work library:
<def:ValueListDef OID="ValueList.SC.SCTESTCD">
<ItemRef ItemOID="SC.SCTESTCD.EDLEVEL" OrderNumber="19" Mandatory="No"/>
<ItemRef ItemOID="SC.SCTESTCD.MARISTAT" OrderNumber="20" Mandatory="No"/>
<ItemRef ItemOID="SC.SCTESTCD.SUBJINIT" OrderNumber="21" Mandatory="No"/>
</def:ValueListDef>
<ItemDef OID="SC.SCTESTCD" Name="SCTESTCD" DataType="text" Length="8"
Origin="Assigned" def:Label="Subject Characteristic Short Name">
<def:ValueListRef ValueListOID="ValueList.SC.SCTESTCD"/>
</ItemDef>
<ItemDef OID="SC.SCTESTCD.EDLEVEL" Name="EDLEVEL" DataType="text"
Length="24" Origin="CRF Page 6" def:Label="Education Level"/>
<ItemDef OID="SC.SCTESTCD.MARISTAT" Name="MARISTAT" DataType="text"
Length="8" Origin="CRF Page 6" def:Label="Marital Status"/>
<ItemDef OID="SC.SCTESTCD.SUBJINIT" Name="SUBJINIT" DataType="text"
Length="3" Origin="CRF Page 3" def:Label="Subject Initials"/>