Special Topic: A Round Trip Exercise Involving the CDISC SDTM and CDISC CRT-DDS Standards
Overview
The typical SAS Clinical
Standards Toolkit workflow in support of the CDISC standards includes
the definition and validation of SDTM submission data and the creation
and validation of a define.xml file based on the SDTM domain data.
This exercise illustrates how you can read a define.xml file to extract
the data and metadata for the purposes of recreating the original
source SDTM study. Recreating the original source study has value
as a stand-alone exercise, either to extract a new SDTM study from
a define.xml file or to create a new SDTM study using information
in a define.xml file as a template.
The Workflow
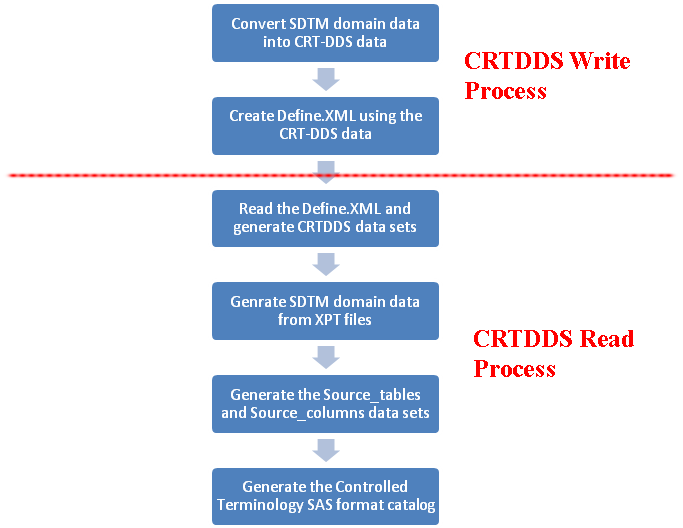
These steps describe
the workflow in more detail. The first five steps describe the derivation
of the CDISC CRT-DDS 1.0 define.xml file.
-
Access a study that contains valid CDISC SDTM data and metadata. This is a study that contains domain data (AE, DM, CO, and so on) and the SAS Clinical Standards Toolkit metadata about that SDTM study, such as source_tables and source_columns. The SAS Clinical Standards Toolkit also includes XSL style sheets, XML map files, and any metadata that is provided by SAS during the SAS Clinical Standards Toolkit installation.
-
Use the set of sample driver programs that are provided in the SAS Clinical Standards Toolkit to define the input and output files for each process task and to invoke the macros that support each standard-specific task. The driver programs are designed to run with the sample studies but can be modified as needed. New custom drivers can also be created and used.
-
Submit the create_crtdds_fromsdtm.sas driver program to access the crtdds_sdtmtodefine macro, and create the 39 data sets that comprise the SAS representation of the CRT-DDS model. These 39 output data sets are written to the
!sasroot /../../SASClinicalStandardsToolkitCRTDDS10/1.4/sample/cdisc-crtdds-1.0/data -
Create the define.xml file by submitting the create_crtdds_define.sas driver program. This driver program generates the define.xml file from the 39 CRT-DDS data sets that were created in step 3. It also calls the crtdds_xmlvalidate macro to validate the XML file structure. The define.xml file is written to the
!sasroot /../../ SASClinicalStandardsToolkitCRTDDS10/1.4/sample/cdisc-crtdds-1.0/sourcexml -
Submit the create_sascrtdds_fromxml.sas driver program. This driver program reads the define.xml file created in step 5, and generates the SAS representation of the CRT¬DDS model using the crtdds_read.sas macro. The data sets created in this step should match the data sets created in step 3. These data sets are written to the
!sasroot /../../SASClinicalStandardsToolkitCRTDDS10/1.4/sample/cdisc-crtdds-1.0/deriveddata!sasroot /../../ SASClinicalStandardsToolkitCRTDDS10/1.4/sample/cdisc-crtdds-1.0/derivedmetadata -
SDTM domain data sets are created based on a reachable set of SAS transport files that are specified in the define.xml file. Submit the create_sasdata_fromxpt.sas SDTM driver program. For SDTM 3.1.2, the program is in the
!sasroot /../../SASClinicalStandardsToolkitSDTM312/1.4/sample/cdisc-sdtm-3.1.2/sascstdemodata/programs!sasroot /../../ SASClinicalStandardsToolkitCRTDDS10/1.4/sample/cdisc-crtdds-1.0/transport!sasroot /../../SASClinicalStandardsToolkitSDTM312/1.4/ sample/cdiscsdtm-3.1.2/sascstdemodata/derived/data -
Source metadata that describes the SDTM domains and columns is derived using information contained in the CRT-DDS data sets derived in step 6. Submit the create_sourcemetadata.sas SDTM driver program. For SDTM 3.1.2, it is installed in the
!sasroot /../../SASClinicalStandardsToolkitSDTM312/1.4/sample/cdisc-sdtm-3.1.2/sascstdemodata/programs!sasroot /../../SASClinicalStandardsToolkitSDTM312/1.4/sample/cdiscsdtm-3.1.2/sascstdemodata/derived/metadata -
SAS formats that support SDTM controlled terminology are derived using information contained in the CRT-DDS data sets that were derived in step 6. Submit the create_formatsfromcrtdds.sas SDTM driver program. For SDTM 3.1.2, this program is installed in the
!sasroot /../../SASClinicalStandardsToolkitSDTM312/1.4/sample/cdisc-sdtm-3.1.2/sascstdemodata/programs!sasroot /../../ SASClinicalStandardsToolkitSDTM312/1.4/sample/cdiscsdtm-3.1.2/sascstdemodata/derived/formats
Once the round trip
exercise is complete, data derived from the process should match the
original data. There might be some metadata collected that does not
match exactly (particularly any date and time fields that collect
real-time information). Differences can be detected by doing a PROC
COMPARE with any of the derived data and metadata data sets against
the original data and metadata data sets.
Running Multiple Driver Programs
CAUTION:
When running
multiple driver programs, be aware that the SAS Clinical Standards
Toolkit uses autocall macro libraries to contain and reference standard-specific
code libraries.
This becomes a problem
with repeated calls to %cstutil_processsetup() or %cstutil_allocatesasreferences
in the same SAS session. You might receive SAS errors, such as this
one, unless you submit some specific SAS code:
ERROR - At least one file associated with fileref SDTMAUTO is still
in use. ERROR - Error in the FILENAME statement.If you call %cstutil_processsetup()
or %cstutil_allocatesasreferences more than once in the same SAS session,
by default the SAS Clinical Standards Toolkit does not attempt to
reallocate SAS librefs and filerefs. Records will be written to the
process results data set noting (for example):
Generally, if you are
resubmitting the same process code again, without changing the &_cststandard
or &_cststandardversion global macro variables, or pointers to
different data or metadata libraries, this is of no consequence. However,
if you are attempting to change the standard or version in the same
SAS session, or you are attempting to reference different studies,
code libraries or terminology libraries, it is imperative that you
use this code between each code submission: