Special Topic: Validation Customization
Overview
Case Study 1: Modifying an Existing Standard or Defining a New Reference Standard
Source data and metadata
are validated in the SAS Clinical Standards Toolkit against a reference
standard. For CDISC standards, the SAS Clinical Standards Toolkit
provides a SAS interpretation of the supported CDISC standards. Because
CDISC standards are guidelines, they are open to interpretation and
customer-specific implementations. Not all clinical studies have all
CDISC-defined standard domains, and most clinical studies have additional
domains reflecting the focus of the clinical study. In addition, CDISC
SDTM domain classes (findings, events, and interventions) enable the
inclusion and exclusion of most columns, depending on the clinical
data points collected in the study. CDISC guidelines generally do
not specify column lengths.
Each of these factors
suggests that the SAS Clinical Standards Toolkit CDISC reference standards
will be modified or replaced with customer-derived standards. The
SAS Clinical Standards Toolkit offers the option of building a reference
standard to encompass domain and column customizations. Or, you can
customize check macros and check logic to perform specific compliance
assessments to a standard. For example, in CDISC SDTM, it is not uncommon
to build multiple supplemental qualifier domains (for example, SUPPAE)
associated with a core reference domain (for example, AE). It is at
the customer's discretion whether the reference standard is modified
to include each unique supplemental qualifier domain, or to use existing
SAS Clinical Standards Toolkit validation check macros with unique
code logic or custom check macros to validate the custom domains.
These latter options are discussed in the following case studies.
It is likely that customers
will derive multiple reference standards. From a SAS Clinical Standards
Toolkit validation perspective, the only relevant reference standard
is the one defined in the SASReferences data set (as type=referencemetadata).
For information
about registering a new standard in the SAS Clinical Standards Toolkit,
see Registering a New Version of a Standard.
Case Study 2: Using Any Set of Source Data and Metadata
From a SAS Clinical
Standards Toolkit perspective, a source study is defined by the study
domains, the study metadata represented in the source_tables and source_columns
data sets, and anything that might be unique to a specific study,
including controlled terminologies, properties, validation checks,
and associated messages.
One key SAS Clinical
Standards Toolkit requirement is that source study elements should
be kept in synchronization. Another key requirement is that all relevant
source study elements should be accurately represented in a SASReferences
data set. The synchronization of study elements is a task that is
often performed outside the SAS Clinical Standards Toolkit. The study
data libraries must contain the domains of interest, the study metadata
must provide the complete set of table-level and column-level metadata
necessary to describe the source data, and any format catalogs and
coding dictionaries supporting the study must be available.
Tip
Best Practice Recommendation:
If a standard folder hierarchy is adopted for source studies, such
as in the SAS Clinical Standards Toolkit CDISC SDTM 3.1.2 sample study
in SAS 9.3 (!sasroot /../../SASClinicalStandardsToolkitSDTM312/1.4/sample/cdisc-sdtm-3.1.2/sascstdemodataCase Study 3: Modifying the SAS Validation Checks for Supported Standards
This case study addresses
adding multiple instances of existing checks. The most common ways
to modify SAS validation checks include:
-
Altering the scope of the domains and columns to be validated. Many checks are defined to be run against specific domains or columns, against specific classes of domains (for example, CDISC SDTM findings, events, or interventions), or against all available domains or columns. As you find it useful to modify a reference standard (for example, to include other domains you consistently use) or you have one or more studies that have new domains, changes are likely to involve alterations to the Validation Master and Validation Control (run time) tablescope or columnscope fields.
-
Changing the Validation Control codelogic field to alter the logic used to identify error conditions. This might be a necessary change if a check needs to be generalized to accommodate new domains or columns. Or, customer conventions might differ from those in the SAS Clinical Standards Toolkit checks.
-
If customer code changes are sufficiently significant, then it might be better to create a new validation check macro. (See Case Study 5: Modifying Existing Validation Check Macros or Adding New Macros.) If a new validation check macro is required, then the Validation Control codesource field needs to be modified to contain the name of the new check macro.
-
The Validation Control uniqueid field provides a way to uniquely identify a specific validation check for reference. Any substantive change to any Validation Control data set check field normally leads to a new uniqueid. For information about the structure of uniqueid, see Column Descriptions of the Validation Master Data Set.
Case Study 4: Adding New Validation Checks for Supported Standards
To add a new validation
check, consider this checklist:
-
Check metadata must conform to the Validation Master structure. (For more information, see Framework.)
-
Certain Validation Master fields accept any user-defined value (for example, checksource, sourceid, checktype, standardref, and checkstatus). These fields are not referenced by the validation check macros. The remaining fields are used in the validation check macros, so you must abide by the SAS Clinical Standards Toolkit conventions. These conventions are described in Framework.
-
For each new validation check, a matching message is required. This is the message that you want written to the Results data set when an error condition is detected. For details, see Messages.
-
Use a similar validation check as a template to build the check metadata required by the SAS Clinical Standards Toolkit. Ask yourself the following types of questions:
-
Look at the Validation Master data set checktype column. Does it look only at table or column metadata, and not at data values (Metadata)? Does it require a specific raw column value (ColumnValue), or a value that complies with some controlled terminology (Cntlterm)? Must the assessment look across multiple records (Multirecord) or multiple tables (Multitable)?
-
-
Does the check apply to a specific class of tables (for example, Class:Findings)? Does the check apply to all tables for the standard (_ALL_)? Does the check apply only to one or more specific tables (for example, DM+TA)? Does the check apply to all tables except one (for example, _ALL_-DM)? Does the check compare the same column in two tables (for example, [DM][TA])?
-
Does the check apply to all columns in the selected tables (_ALL_)? Does the check apply only to one column (for example, USUBJID)? Does the check compare two columns in the same table (for example, [AESDTH][AEOUT])? Does the check apply to all column names that end in a particular suffix (for example, **DTC)?
-
-
Case Study 5: Modifying Existing Validation Check Macros or Adding New Macros
The SAS Clinical Standards
Toolkit provides 18 validation check macros. These macros, located
in the primary SAS Clinical Standards Toolkit autocall library, offer
a variety of code examples that are available to all standards supporting
validation. For information
about the purpose and use of each check macro, see Special Topic: Validation Check Macros and Macro Application Programming Interface.
Some validation scenarios
might require modifications to the SAS Clinical Standards Toolkit
check macros or the derivations of new macros. If so, these guidelines
should be followed. These guidelines facilitate the use of these macros
in the general SAS Clinical Standards Toolkit framework and in the
specific SAS Clinical Standards Toolkit validation framework.
-
Conform to the basic check macro workflow. This workflow is described in Special Topic: Validation Check Macros.
Case Study 6: Modifying the SAS Clinical Standards Toolkit Messaging, Including Internationalization
This case study considers
these three issues related to the support of the SAS Clinical Standards
Toolkit messaging:
A SAS Clinical Standards
Toolkit message is created for each distinct combination of the Validation
Master standard and checksource fields. This allows the SAS Clinical
Standards Toolkit to support checksource-specific messaging and severity.
A unique SAS Clinical Standards Toolkit message is required for each
value of the Validation Master standardversion field if that value
is not the wildcard ***.
The SAS Clinical Standards
Toolkit representation of the SDTM0013 check in the Messages data
set is:
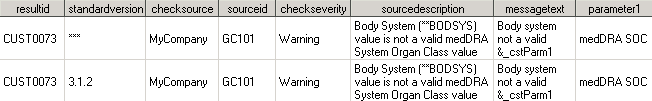
Messages Data Set Excerpt for Check SDTM0013

The Messages data set
contains two records because there are two distinct checksource values
for Validation Master checkid SDTM0013.
Three separate invocations
of CUST0073 are represented. Each record points to a different domain
(tablescope). This example assumes that the CDISC SDTM 3.1.2 standard
has been registered. The first and third records (AE and MH domains)
indicate that this specific implementation of the check is applicable
to all versions of CDISC SDTM. However, the second record is applicable
to only CDISC SDTM 3.1.2 (because CE is a new domain in SDTM 3.1.2).
It is the distinct combinations
of the Validation Master checkid, standardversion, and checksource
fields that control the associated Messages data set records.
It is important to maintain
the relationship between messages and validation check macro code.
If the validation check macro code references an unknown resultid,
the text
<Message lookup failed to find matching record> is written to the Results data set.
The CUST0073 check defines
a substitution parameter (&_cstParm1). (The SAS Clinical Standards
Toolkit code assumes that message substitution parameters begin with
the string &_cst.) For the calling validation check macro to support
parameters when writing output to the Results data set, the parameters
that are passed should be syntactically consistent with the messagetext
field in the Messages data set.
Building the message
record to use a default value (as specified in the parameter1 field)
solves the problem when the calling macro fails to pass a substitution
value. Using parameters is optional. Parameters might be needed only
if the message is to be used in multiple contexts where substitutions
of parameter values help interpret the message.
The SAS Clinical Standards
Toolkit supports the internationalization of messages through specifying
message file references in the SASReferences data set (type=messages).
If referenced message files conform to the structure expected by the
SAS Clinical Standards Toolkit, any text, including internationalized
text, can be included.
Case Study 7: Validation of Multiple Studies
Most illustrations and
discussions in this chapter assume a reference to a single clinical
study. But, what if you need to validate multiple clinical studies
at one time? A key consideration is the information that source data
libraries and source metadata files contain, and how they should be
referenced in the SASReferences data set used by the validation process.
Consider the following
four methodologies, which are ordered based on estimated rates of
adoption. Other candidate methodologies are possible.
-
A common methodology is to build single source data and metadata libraries that contain pooled data sets where metadata reconciliation has already occurred. (This is frequently done in integrated summaries of efficacy and safety.) In this case, the SASReferences data set will contain a single type=sourcedata record pointing to the pooled integrated data library. The SASReferences SAS librefs (where type=sourcemetadata) must match the source metadata library references in the sasref column of the table and column metadata data sets.
-
A second methodology is to build a SAS Clinical Standards Toolkit process that daisy-chains multiple job streams, where each study is defined in a unique SASReferences data set and validated independently. Within the same SAS session, unless your validation process deletes work files, the results and metrics files are appended. The files at the end of the process contain results for all studies.
-
An alternative approach defines a single SASReferences libref for multiple type=sourcedata records, each pointing to a different study source library. The SAS Clinical Standards Toolkit supports library concatenation, but SAS only reads data sets from the first defined library when the same data set name occurs in multiple libraries. Because standard domain names are expected, this approach does not work unless a unique domain-naming convention across studies is used. A similar approach is required for source metadata. These constraints make this approach less tenable.
-
Another alternative methodology is to use multiple SASReferences librefs (multiple type=sourcedata records). You have one for each study source library, and a single source metadata library (with one table and one column metadata data set, setting the SASRef column to each libref used in SASReferences). This methodology works for any validation check that does not compare columns across domains or compares domains. Because of the way tablescope and columnscope parsing occurs in the SAS Clinical Standards Toolkit, source data libraries are not considered. This makes it possible that unintended comparisons of multiple columns or multiple domains from different studies can occur. As a result, this methodology is not recommended.