Maintenance Usage Scenarios
Overview
The following sections
describe usage scenarios that the framework accommodates. Code that
is required to complete the usage scenario included in each section.
All macros that are provided in the usage scenarios are in the primary
SAS Clinical Data Standards Toolkit autocall path:
Note: All of the maintenance usage
scenarios require that you have Write access to the Global Standards
Library.
For complete
macro documentation, see Macro Application Programming Interface.
Registering a New Version of a Standard
This code defines and
registers a new standard. The code can also be used to register a
new version of an existing standard.
/*
Step 1. Ensure that the macro variable pointing to the global standards
library exists.
*/
%cstutil_setcstgroot;
/*
Step 2. Register the standard with the Toolkit global standards
library
*/
%cst_registerstandard(
_cstRootPath=%nrstr(&_cstGRoot./standards/myStandard),
_cstControlSubPath=control,
_cstStdDSName=standards,
_cstStdSASRefsDSName=StandardSASReferences);
The
_cstRootPath parameter uses %nrstr(&_cstGroot) so
that the &_cstGroot is registered as
a macro variable. This specification allows the global standards library
to be moved or copied without reregistering the full path of the new
standard.
When defining
and registering a new standard, you should evaluate which of the metadata
files described in Common Framework Metadata should be provided to support new standard functionality.
For example:
For more information
about the metadata files that support the SAS Clinical Standards Toolkit,
see Metadata File Descriptions. You can define new metadata types. These new
metadata types should be documented in the standard-specific StandardSASReferences
and Standardlookup data sets, and in the SAS Clinical Standards Toolkit
framework Standardlookup data set.
Setting the Default Version for a Standard
When multiple versions
of a standard exist, the first version that is installed is set as
the default. The default version is used when multiple versions of
a standard have been registered, and a specific version is not provided
in a macro call or in a SASReferences file. This code modifies the
default version of a specific standard:
%cst_setstandardversiondefault(
_cstStandard=CDISC-SDTM
,_cstStandardVersion=3.1.1
);
Unregistering a Standard Version
Unregistering an Old Version of a Standard, and Then Registering a New Version of a Standard
Suppose that the SAS
Clinical Standards Toolkit 1.3 is currently installed and used. The
SAS Clinical Standards Toolkit 1.4 is released. You want the product
updates for a standard version. In the following steps, the CDISC
SDTM standard is used as an example. However, the steps apply to all
other standard versions. You want to set version 3.1.2 as the default
version for the CDISC SDTM standard. The SAS Clinical Standards Toolkit
installation process does not do this automatically because you might
have made updates to the SAS Clinical Standards Toolkit 1.3 code base
or metadata that you want to preserve. Or, you might want to test
the SAS Clinical Standards Toolkit 1.4 CDISC SDTM 3.1.2 implementation
before declaring it the new default version.
Step 1: Confirm that
multiple versions of the standard are available. Confirm that registration
of a new version is needed.
-
Confirm which revision of the standard-version is currently in use.
-
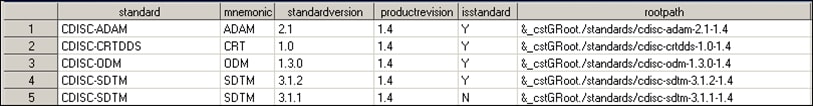
Open the Standards data set in the library, and confirm that the older version is the one being used.This display shows that the registered version CDISC SDTM 3.1.1.-1.3 indicates that it is the original version that was shipped with the SAS Clinical Standards Toolkit 1.3. It is defined as the default version for the CDISC SDTM standard.Global Standards Library Metadata Standards Data Set before Updates
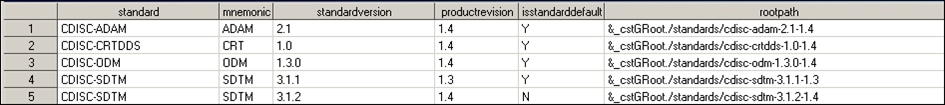
Step 2: Register the
updated CDISC SDTM 3.1.1 metadata in the global standards library
to use the SAS Clinical Standards Toolkit 1.4.
-
%cstutil_setcstgroot; /* Set the framework properties used for the uninstall */ %cst_setstandardproperties( _cstStandard=CST-FRAMEWORK, _cstSubType=initialize ); /* If the version to be replaced is the default, you must make another version the default. In this case, this is the desired final outcome anyway. */ %cst_setstandardversiondefault( _cstStandard=CDISC-SDTM ,_cstStandardVersion=3.1.2 ); /* Unregister the standard */ %cst_unregisterstandard( _cstStandard=CDISC-SDTM ,_cstStandardVersion=3.1.1 );