Running a Validation Process
Sample CDISC SDTM 3.1.1 Driver Program: validate_data.sas
Each SAS
Clinical Standards Toolkit process uses a SAS driver module to set
up the program execution flow. The following steps show the execution
flow in a typical SAS driver module to perform SAS Clinical Standards
Toolkit validation. For example, in a SAS 9.2 deployment, the CDISC
SDTM 3.1.2 validation driver module can be found in:
!sasroot /../../SASClinicalStandardsToolkitSDTM312/1.3/sample/cdisc-sdtm-3.1.2/sascstdemodata/programs/validate_data.sas/* There are several ways to define the study data and metadata
locations. These include (but are not limited to):
- Pre-allocation of libraries through some user-defined set-up mechanism
- Definition within a user-defined driver program such as this one
- Full explicit definition within a work sasreferences control data set
- Use of a global macro variable referenced within each sasreferences file
This driver program illustrates use of the last mechanism, setting the
global macro variables studyRootPath and studyOutputPath, which are referenced
within the sample study sasreferences data set path column.
Note this example is dependent on the SAS version and installation folder structure. */
data _null_;
select("&sysver");
when("9.1")
do;
call symput('studyRootPath','!sasroot/../SASClinicalStandardsToolkitSDTM312
/1.3/sample/cdisc-sdtm-3.1.2/sascstdemodata');
call symput('studyOutputPath','!sasroot/../SASClinicalStandardsToolkitSDTM312
/1.3/sample/cdisc-sdtm-3.1.2/sascstdemodata');
end;
otherwise do;
call symput('studyRootPath','!sasroot/../../SASClinicalStandardsToolkitSDTM312
/1.3/sample/cdisc-sdtm-3.1.2/sascstdemodata');
call symput('studyOutputPath','!sasroot/../../SASClinicalStandardsToolkitSDTM312
/1.3/sample/cdisc-sdtm-3.1.2/sascstdemodata');
end;
end;
run;The &studyRootPath
and &studyOutputPath variables facilitate code standardization
and portability. They are not required.
The workPath
value provides the path for the Work directory. This directory is
referenced within the sample study SASReferences data set path column.
It is not required.
* Note the number of calls should match the unique studyOutputPath subdirectories in sasreferences *; %****cstutil_createunixsubdir(_cstSubDir=results); * <--- example UNIX override *;
The SAS
Clinical Standards Toolkit processes normally create one or more output
files. These files might reside in the Work directory or point to
some external location. The &studyRootPath variable points to
read-only locations in the !sasroot folder hierarchy. The &studyOutputPath
variable points to writable locations for process output, often in
the !sasroot folder heirarchy.
UNIX users (or any users) might find it necessary to reset &studyOutputPath
to some write-enabled location since the !sasroot directories are
typically write protected. For these users, calls to the %cstutil_createunixsubdir
macro create any workpath subdirectories that are expected by SASReferences
records and set &studyOutputPath to workpath.
These
convenience macro variables are used primarily for reporting purposes
later in the validation process.
Step 2: Set the SAS Clinical Standards Toolkit framework properties
and global macro variables. Create an empty work.sasreferences data
set to be populated in the validation process.
* Set properties provided as part of the CST-FRAMEWORK standard. ; %cst_setStandardProperties( _cstStandard=CST-FRAMEWORK,_cstSubType=initialize); %cst_createds(_cstStandard=CST-FRAMEWORK, _cstType=control,_cstSubType=reference, _cstOutputDS=work.sasreferences);
Each registered
standard should have its own initialize.properties. For each standard
that is included in a specific process, the %cst_setStandardProperties
macro can be called at this point. Alternatively, type=properties
records can be added to the SASReferences data set, and properties
are processed when the %cstutil_allocatesasreferences macro is called.
This latter approach is followed in the SDTM validate_data.sas driver
module.
The validate_data.sas
module initializes the SASReferences data set that is required for
SDTM validation. The SASReference data set defines the location and
name of the Validation Control data set. The Validation Control data
set contains the set of checks to be included in the validation process.
The sample validate_data.sas driver progra, sets the path of the Validation
Control data set to
&studyRootPath/control and name to validation_control.sas7bdat. In SAS 9.2, this translates to !sasroot/../../ SASClinicalStandardsToolkitSDTM312/1.3/sample/cdisc-sdtm-3.1.2/sascstdemodata/control/validation_control.sas7bdat. For an explanation of the purpose and content of each SASReferences
file, see SASReferences File. For a fully initialized SASReferences data set for SDTM
validation, see Sample SASReferences File for CDISC SDTM Validation.
The %cstutil_processsetup
macro completes process setup. It ensures that all SAS librefs and
filerefs are allocated; all system options, macro autocall paths and
format search paths are set; and that all global macro variables that
are required by the process have been appropriately initialized.
The %cstutil_processsetup
macro uses the following parameters.
This parameter determines
what initial source setup should be based on. Valid values are SASREFERENCES
(default) or RESULTS. If RESULTS is specified, then no other parameters
are required, and setup responsibility is passed to the cstutil_reportsetup
macro. The Results data set name must be passed to cstutil_reportsetup
as libref.memname.
The %cstutil_processsetup
macro call:
%cstutil_processsetup();in the validate_data.sas driver reflects the acceptance of the macro parameter defaults listed above.
The %cstutil_processsetup
macro parameter values tell the process where to find the SASReferences
data set.
*********************************************************************; * Set global macro variables for the location of the sasreferences *; * file (overrides default properties initialized above *; *********************************************************************; %let _cstSASRefsName=&_cstSASReferencesName; %let _cstSASRefsLoc=&_cstSASReferencesLocation;
The final
setup step for the %cstutil_processsetup macro is a call to the %cstutil_allocatesasreferences
utility macro. The SASReferences data set is now interpreted by the
SAS Clinical Standards Toolkit. The following actions complete the
process:
-
The %cst_insertstandardsasrefs macro is called to insert paths into any records that are missing path information. The information is captured from the StandardSASReferences data set for each standard. For more information about how this works, see Inserting Information from Registered Standards into a SASReferences File.
At this
point, all libraries should be allocated, all paths and global macros
should be set, and the global status macro variable _cst_rc should
be set to 0. The process is ready to proceed.
This is
a common process failure point because of the importance of the SASReferences
data set. The SASReferences data set is key to the process, and any
errors will cause the process to fail. For tips on debugging problems
with the SASReferences data set, see Special Topic: Debugging a Validation Process.
For tips
on debugging if unexpected errors occur, see Special Topic: Debugging a Validation Process.
* Clean up the SAS Clinical Standards Toolkit process
files, macro variables and macros.;
%*cstutil_cleanupcstsession(
cstClearCompiledMacros=0
,cstClearLibRefs=0
,cstResetSASAutos=0
,cstResetFmtSearch=0
,cstResetSASOptions=1
,cstDeleteFiles=1
,cstDeleteGlobalMacroVars=0);
Step 6
is optional, and it is unnecessary with batch processing. You should
not clean up prematurely or aggressively if additional SAS Clinical
Standards Toolkit processes are to be run in the same interactive
SAS session.
The following
table summarizes what the SAS Clinical Standards Toolkit attempts
to do when each of the %cstutil_cleanupcstsession macro parameters
is enabled:
Parameter Details for the %cstutil_cleanupcstsession Macro
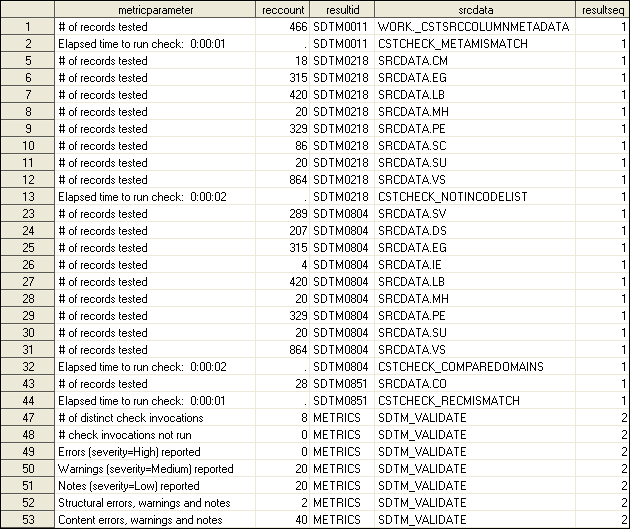
Validation Results and Metrics
For SAS
Clinical Standards Toolkit validation processes, the primary products
of each validation process are the Results data set and the Metrics
data set. These data sets itemize and summarize the findings of the
validation process.
The following
displays summarize a sample validation process. A few facts about
the sample validation process follow:
-
It included SASReferences records to persist the results as results.validation_results and results.validation_metrics.Comments about the Validation Results Data Sets in Displays 6.9 and 6.10Comments About the Validation Metrics Data SetA summary metric of unique check invocations. Validation Metrics Data Set does not itemize all eight checks.
Note: In Validation Results Data Set (#1) and Validation Results Data Set (#2), some records in the validation Results data
set have been deleted for brevity. This creates an inconsistency with
the metrics listed in Validation Metrics Data Set.
The following
are some general observations:
-
The seqno field is intended to be a record (message) counter in each specific check invocation. Generally, this value starts with 1 on the first record, and increments by 1 until the last record for each checkid and resultseq combination. One exception is with the Validation Control column reportAll=N. This signals the code to not write a record to the Results data set for each record in error. However, seqno continues to increment in this case, resulting in a gap in seqno values, with the last seqno approximating the total number of records in error.
A set
of sample validation reports is available to summarize the SAS Clinical
Standards Toolkit validation process results and metrics. For
more information, see Reporting.
Sample CDISC CRT-DDS 1.0 Driver Program: validate_crtdds_data.sas
SAS Clinical
Standards Toolkit validation of the SAS representation of the CDISC
CRT-DDS standard follows the same pattern used for CDISC SDTM validation.
A sample driver module—validate_crtdds_data.sas—is provided
to perform process setup steps and to call the crtdds_validate.sas
macro. For a more complete description of the validation of the SAS
representation of the CDISC CRT-DDS standard, see XML-Based Standards. In this chapter, the use of the validate_crtdds_data driver
module is described.