The HPNLMOD Procedure
Least Squares Model
The Michaelis-Menten model of enzyme kinetics (Ratkowsky 1990, p. 59) relates a substrate’s concentration to its catalyzed reaction rate. The Michaelis-Menten model can be analyzed using a least squares estimation because it does not specify how the reaction rate is distributed around its predicted value. The relationship between reaction rate and substrate concentration is
![\[ f(\mb{x}, \btheta ) = \frac{\theta _1 x_ i}{\theta _2 + x_ i}, \quad \mbox{for} \, \, i = 1, 2, \ldots , n \]](images/statug_hpnlin0001.png)
where 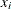 represents the concentration for n trials and
represents the concentration for n trials and 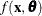 is the reaction rate. The vector
is the reaction rate. The vector 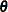 contains the rate parameters.
contains the rate parameters.
For this model, which has experimental measurements of reaction rate and concentration stored in the enzyme data set, the following SAS statements estimate the parameters 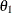 and
and 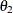 :
:
proc hpnlmod data=enzyme; parms theta1=0 theta2=0; model rate ~ residual(theta1*conc / (theta2 + conc)); run;
The least squares estimation performed by PROC HPNLMOD for this enzyme kinetics problem produces the analysis of variance table that is displayed in Figure 56.1. The table displays the degrees of freedom, sums of squares, and mean squares along with the model F test.
Figure 56.1: Nonlinear Least Squares Analysis of Variance
Finally, Figure 56.2 displays the parameter estimates, standard errors, t statistics, and 95% confidence intervals for 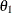 and
and 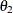 .
.
Figure 56.2: Parameter Estimates and Approximate 95% Confidence Intervals
In the enzyme kinetics model, no information was supplied about the distribution of the reaction rate around the model’s mean value. Therefore, the residual model distribution was specified to perform a least squares parameter fit.
