Reading XML Files
Overview
Support of CDISC XML-based
standards, such as CDISC Define-XML 2.0, CDISC CRT-DDS (define.xml),
and CDISC ODM, includes the ability to read XML files into SAS data
set format. In the SAS Clinical Standards Toolkit, you can read these
types of files:
-
a CDISC CRT-DDS 1.0
-
a CDISC Define-XML 2.0 define.xml file (including Analysis Results Metadata 1.0)
-
a CDISC ODM 1.3.0 or CDISC ODM 1.3.1 XML file
-
the Controlled Terminology files as they are published by the NCI in ODM XML format
Basic Workflow
Here is the basic workflow
for reading XML files:
-
Determine the existence of a valid XML file.
-
Use valid XSL style sheets for each target data set (such as ItemDefs.xsl).
-
Use the SAS DATA step component JavaObj to create a standardized intermediate cubeXML file using the XSL style sheets.
-
Read the standardized cubeXML file using the SAS XML LIBNAME engine and XMLMAP processing.
This basic workflow
is used by all XML-based standards that are supported by the SAS Clinical
Standards Toolkit.
Reading CDISC ODM XML Files: %ODM_READ Macro
Note: The process for reading ODM
XML files is the same for all ODM versions that are supported by the
SAS Clinical Standards Toolkit. The process is explained using ODM
version 1.3.0.
To read an ODM XML file,
a specialized macro named %ODM_READ is available in the ODM 1.3.0
standards macro folder. This folder is located here:
global standards library directory/standards/cdisc-odm-1.3.0-1.7/macrosThis macro is referenced
from the create_sasodm_fromxml.sas driver program (described more
fully below).
File references and
other metadata that are required by the macro are set as global macro
variable values. Currently, these global macro variable values are
set through the framework initialization properties and the CDISC
ODM 1.3.0 initialization properties. Throughout the processing of
the %ODM_READ macro, the Results data set contains all framework and
ODM 1.3.0 specific messages generated during run time.
Based on file references
defined in the SASReferences data set, the %ODM_READ macro accesses
the ODM XML file.
Here is a partial listing
of a sample ODM XML file:
<?xml version="1.0" encoding="ISO-8859-1"?>
<ODM
xmlns="http://www.cdisc.org/ns/odm/v1.3"
FileOID="Study1234"
ODMVersion="1.3"
FileType="Snapshot"
CreationDateTime="2004-07-28T12:34:13-06:00"
SourceSystem="ss00"
AsOfDateTime="2004-07-29T12:34:13-06:00"
Granularity="SingleSite"
Description="Study to determine existence of ischemic stroke"
Archival="Yes"
PriorFileOID="Study-4321"
Originator="SAS Institute"
SourceSystemVersion="Version 0.0.0"
Id="DSSignature123">
<Study OID="1234"
<GlobalVariables>
<StudyName>1234</StudyName>
<StudyDescription>1234 Data Definition</StudyDescription>
<ProtocolName>1234</ProtocolName>
</GlobalVariables>
<MeasurementUnit OID="MeasurementUnits.OID.MMHG" Name="MMHG"
<Symbol>
<TranslatedText xml:lang="en">mmHG</TranslatedText>
<TranslatedText xml:lang="fr-CA">mmHG</TranslatedText>
</Symbol>
</MeasurementUnit>
<MeasurementUnit OID="MeasurementUnits.OID.YRS" Name="YEARS">
<Symbol>
<TranslatedText xml:lang="de">Jahren</TranslatedText>
<TranslatedText xml:lang="en">Years of age</TranslatedText>
<TranslatedText xml:lang="fr-CA">Ans</TranslatedText>
</Symbol>
</BasicDefinitions>
<MetaDataVersion MetaDataVersion OID="CDISC.SDTM.3.1.0"
Name="Study 1234, Data Definitions"
Description="Study 1234, Data Definitions">
<Include StudyOID="1234" MetaDataVersionOID="MDV000">
</Include>
<Protocol>
<Description>
After the %ODM_READ
macro confirms that the ODM XML file exists, a call is made to the
SAS DATA step component JavaObj. JavaObj processing converts the ODM
XML file into the cubeXML file through transformations using XSL files
and processes. The cubeXML file is created in the Work library. The
name of the cubeXML file is _cubnnnn.xml,
where nnnn is a randomly
generated number. The cubeXML file is accessed using the SAS XML LIBNAME
engine and XMLMAP processing. A default XMLMAP file is stored in the
sample ODM 1.3.0 study folder hierarchy under
/referencexml as odm.map. The odm.map file is required to process
the cubeXML file. If it does not exist, then the %ODM_READ macro attempts
to create one using the ODM reference metadata.
Here is a partial listing
of the odm.map file.
<?xml version="1.0" encoding="windows-1252"?>
<SXLEMAP name="ODM130" version="1.2">
<TABLE name="ItemDefs">
<TABLE-PATH syntax="XPath">/LIBRARY/ItemDefs</TABLE-PATH>
<TABLE-DESCRIPTION>Item metadata</TABLE-DESCRIPTION>
<COLUMN name="OID">
<PATH syntax="Xpath">/LIBRARY/ItemDefs/OID</PATH>
<TYPE>character</TYPE>
<DATATYPE>character</DATATYPE>
<DESCRIPTION>Unique identifier for this item</DESCRIPTION>
<LENGTH>64</LENGTH>
</COLUMN>
<COLUMN name="Name">
<PATH syntax="Xpath">/LIBRARY/ItemDefs/Name</PATH>
<TYPE>character</TYPE>
<DATATYPE>character</DATATYPE>
<DESCRIPTION>Item (variable) name</DESCRIPTION>
<LENGTH>128</LENGTH>
</COLUMN>
<COLUMN name="DataType">
<PATH syntax="Xpath">/LIBRARY/ItemDefs/DataType</PATH>
<TYPE>character</TYPE>
<DATATYPE>character</DATATYPE>
<DESCRIPTION>Item (variable) data type (text, integer, float)</DESCRIPTION>
<LENGTH>18</LENGTH>
</COLUMN>
<COLUMN name="Length">
<PATH syntax="Xpath">/LIBRARY/ItemDefs/Length</PATH>
<TYPE>numeric</TYPE>
<DATATYPE>numeric</DATATYPE>
<DESCRIPTION>Item (variable) length</DESCRIPTION>
<LENGTH>8</LENGTH>
</COLUMN>When the cubeXML is
processed, each of the 66 data sets (such as ItemDefs) that are included
in the SAS representation of the CDISC ODM 1.3.0 model is derived.
Note: For more information about
the %ODM_READ macro, see the SAS Clinical Standards Toolkit: Macro API Documentation.
By default, if a null-parameter
%ODM_READ macro call is made, source metadata files and SAS format
catalogs for each language found in the clitemdecodetranslatedtext
data set are created after the SAS data sets representing the ODM
XML metadata and data content are derived. The target location of
the derived metadata files is defined in the SASReferences data set.
The target location of any derived SAS format catalogs is the SAS
Work library unless defined in the SASReferences data set.
Sample Driver Program: create_sasodm_fromxml.sas
Overview
Each primary SAS Clinical
Standards Toolkit task, such as reading CDISC ODM XML files, is guided
by a sample driver program that is provided with the SAS Clinical
Standards Toolkit. For reading ODM XML files, this program is create_sasodm_fromxml.sas.
The driver program is
located here:
sample study library directory/cdisc-odm-1.3.0–1.7/programsThe SASReferences Data Set
As a part of
each SAS Clinical Standards Toolkit process setup, a valid SASReferences
data set is required. It references the input files that are needed,
the librefs and filenames to use, and the names and locations of data
sets to be created by the process. It can be modified to point to
study-specific files. For
an explanation of the SASReferences data set, see SASReferences File.
In the SASReferences
data set, there are two input file references and five output data
set references that are key to the successful completion of the driver
program. Key Components of the SASReferences Data Set for the create_sasodm_fromxml.sas Driver
Program lists these
files and data sets, and they are discussed in separate sections. In the sample create_sasodm_fromxml.sas driver program,
these values are set for &studyRootPath and &studyOutputPath:
&studyRootPath=&_cstSRoot/cdisc-odm-&_cstStandardVersion.-&_cstVersion&studyOutputPath=&_cstSRoot/cdisc-odm-&_cstStandardVersion.-&_cstVersion|
Metadata Type
|
SAS LIBNAME or Fileref
to Use
|
Reference Type
|
Path
|
Name of File
|
|---|---|---|---|---|
|
Input
|
||||
|
externalxml
|
odmxml
|
fileref
|
&studyRootPath/sourcexml
|
odm_sample.xml
|
|
referencexml
|
odmmap
|
fileref
|
&studyRootPath/referencexml
|
odm.map
|
|
Output
|
||||
|
sourcedata
|
srcdata
|
libref
|
&studyOutputPath/derived/data
|
*.*
|
|
sourcemetadata
|
srcmeta
|
libref
|
&studyOutputPath/derived/metadata
|
source_tables.sas7bdat
|
|
sourcemetadata
|
srcmeta
|
libref
|
&studyOutputPath/derived/metadata
|
source_columns.sas7bdat
|
|
targetdata
|
trgdata
|
libref
|
&studyOutputPath/derived/formats
|
|
|
results
|
results
|
libref
|
&studyOutputPath/results
|
read_results.sas7bdat
|
Process Inputs
The externalxml type
refers to the ODM XML file to read. The filename reference odmxml
is defined in the SASReferences data set. This filename reference
is used in the submitted SAS code when referring to the ODM XML file.
The referencexml type
refers to the SAS map file that is used to generate the SAS data sets
that represent the ODM file metadata and content. The filename reference
odmmap is defined in the SASReferences data set. This filename reference
is used in the submitted SAS code when referring to the SAS map file.
If a path and filename for the map file are not specified, a temporary
map file is created as part of the odm_read processing.
Process Outputs
When the driver program
finishes running, the read_results data set is created in the Results
library. This data set contains informational, warning, and error
messages that were generated by the driver program.
The following display
shows an example of the contents of a Results data set that was created
while reading the sample ODM XML file that was provided with the SAS
Clinical Standards Toolkit:
Example of a Partial Results Data Set Created by the create_sasodm_fromxml.sas
Driver Program
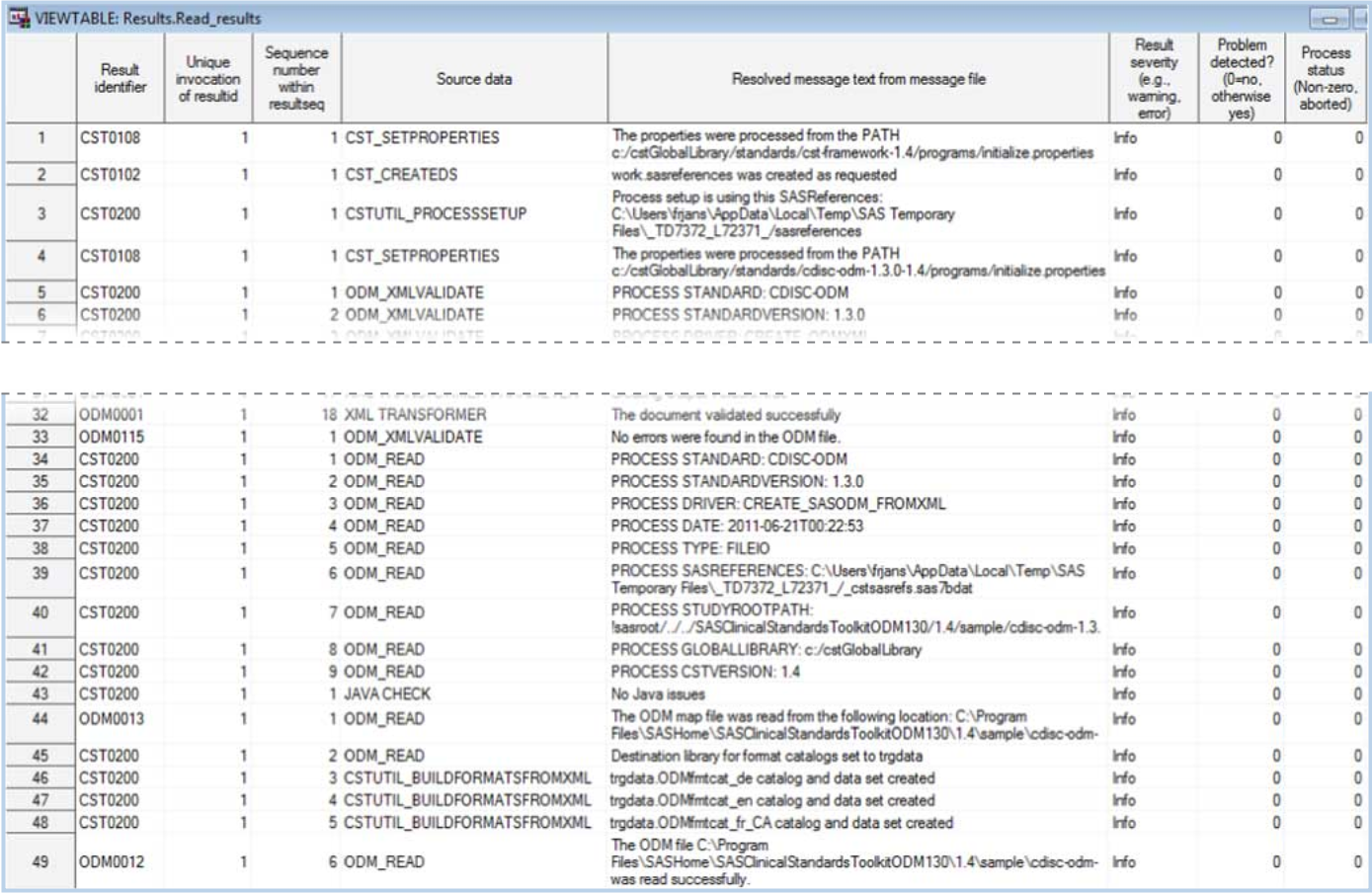
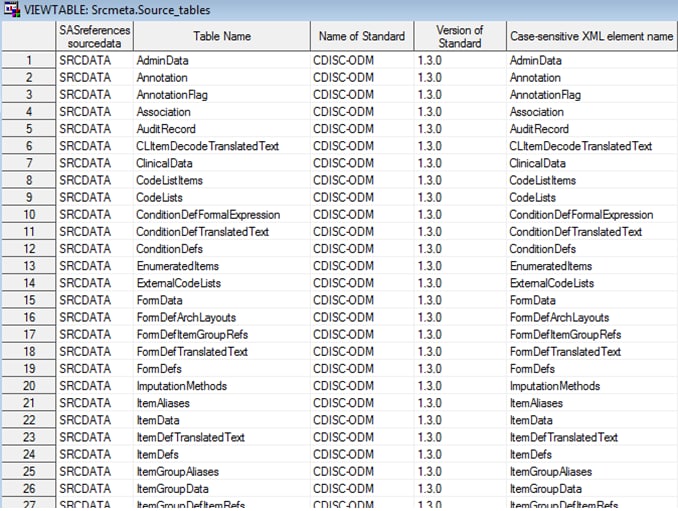
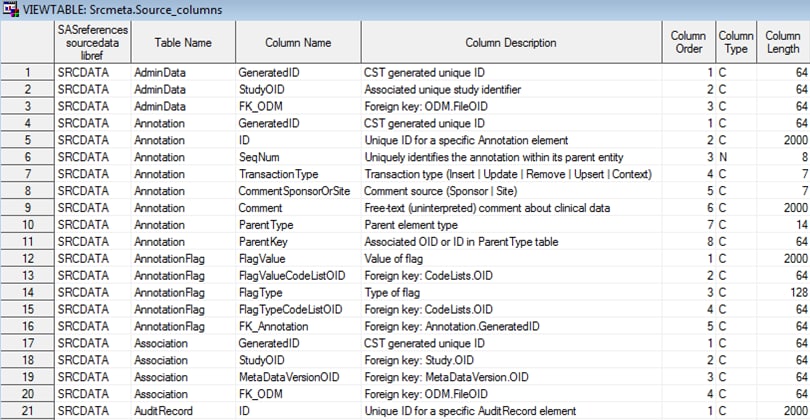
The %ODM_READ macro
creates the source_tables and source_columns data sets in the Srcmeta
library. These data sets contain the table and column metadata for
each of the SAS data sets that is derived from the ODM XML file.
Example of Partial Source_Tables Data Set Derived from the
%ODM_READ Macro
Example of Partial Source_Columns Data Set Derived from the
%ODM_READ Macro
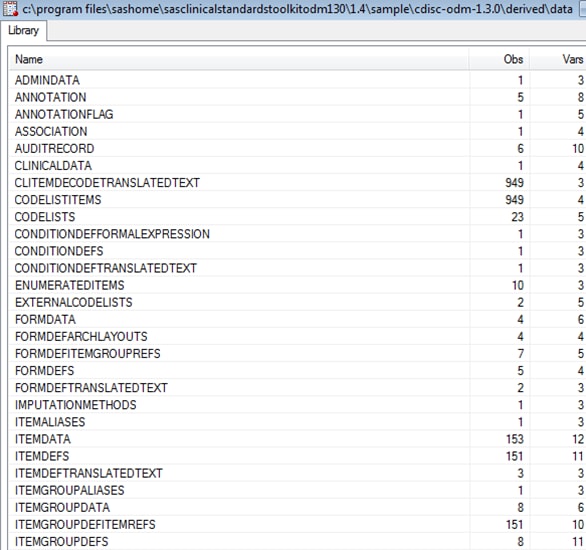
The Srcdata library
contains the SAS data sets that represent the ODM file metadata and
content. By default, the %ODM_READ macro creates 66 unique data sets
in the SAS Clinical Standards Toolkit for ODM 1.3.0. Some of these
data sets might be empty if no associated content was derived from
the ODM XML file. There is a one-to-one correspondence between the
tables listed in the Srcdata library and the tables contained in the
source_tables metadata file in the Srcmeta library.
Example of Partial Srcdata Library Derived from the %ODM_READ
Macro
Extracting Clinical Data and Reference Data from the SAS Representation of an ODM XML File: %ODM_EXTRACTDOMAINDATA Macro
As the primary interchange
format for CDISC, ODM XML is a common format for electronic data capture
(EDC) data management views of clinical data. This format often does
not closely approximate submission (SDTM) and analysis (ADaM) data
structures unless the EDC views have been built using the CDISC CDASH
standard. From a SAS perspective, you might want to extract clinical
data from an ODM XML file to serve as source data for transformations
that derive SDTM domain data sets.
The %ODM_EXTRACTDOMAINDATA
macro supports extracting clinical data or reference data from the
SAS data sets that were created by the %ODM_READ macro.
The %ODM_EXTRACTDOMAINDATA
macro makes the following assumptions:
-
An ODM XML file is available that contains sufficient metadata and content for extractable clinical data and reference data.
-
A full SAS representation of an ODM XML file is available (for example, the %ODM_READ macro has been run against the XML file).
-
The SAS representation of an ODM XML file contains both metadata and data.By default, the driver assumes all source data files reside in the sample derived folder or the data folder that is typically populated by running the %ODM_READ macro. However, the source data files and the source metadata files can be in different folders.
-
Any codelists defined in the ODM XML file and associated with extracted data set columns are available as part of the output of the %ODM_READ macro.
ODM integer and float
data types are converted to SAS numeric data. All other ODM data types
are converted to SAS character data. If an integer or float data value
cannot be converted, a warning appears in the SAS log and Results
data set.
Here is a partial listing
of the metadata in a sample ODM XML file:
<ItemGroupDef OID="ItemGroupDefs.OID.AE" Repeating="Yes"
SASDatasetName="AE" Name="Adverse Events" Domain="AE"
Comment="Some adverse events from this trial">
<ItemRef ItemOID="ID.TAREA" OrderNumber="1" Mandatory="No" />
<ItemRef ItemOID="ID.PNO" OrderNumber="2" Mandatory="No" />
<ItemRef ItemOID="ID.SCTRY" OrderNumber="3" Mandatory="No" />
<ItemRef ItemOID="ID.F_STATUS" OrderNumber="4" Mandatory="No" />
<ItemRef ItemOID="ID.LINE_NO" OrderNumber="5" Mandatory="No" />
<ItemRef ItemOID="ID.AETERM" OrderNumber="6" Mandatory="No" />
<ItemRef ItemOID="ID.AESTMON" OrderNumber="7" Mandatory="No" />
<ItemRef ItemOID="ID.AESTDAY" OrderNumber="8" Mandatory="No" />
<ItemRef ItemOID="ID.AESTYR" OrderNumber="9" Mandatory="No" />
<ItemRef ItemOID="ID.AESTDT" OrderNumber="10" Mandatory="No" />
<ItemRef ItemOID="ID.AEENMON" OrderNumber="11" Mandatory="No" />
<ItemRef ItemOID="ID.AEENDAY" OrderNumber="12" Mandatory="No" />
<ItemRef ItemOID="ID.AEENYR" OrderNumber="13" Mandatory="No" />
<ItemRef ItemOID="ID.AEENDT" OrderNumber="14" Mandatory="No" />
<ItemRef ItemOID="ID.AESEV" OrderNumber="15" Mandatory="No" />
<ItemRef ItemOID="ID.AEREL" OrderNumber="16" Mandatory="No" />
<ItemRef ItemOID="ID.AEOUT" OrderNumber="17" Mandatory="No" />
<ItemRef ItemOID="ID.AEACTTRT" OrderNumber="18" Mandatory="No" />
<ItemRef ItemOID="ID.AECONTRT" OrderNumber="19" Mandatory="No" />
</ItemGroupDef>
...
<ItemDef OID="ID.AESTDT" SASFieldName="AESTDT"
Name="Derived Start Date" DataType="date"/>
<ItemDef OID="ID.AEENMON" SASFieldName="AEENMON"
Name="Stop Month - Enter Two Digits 01-12" DataType="integer" Length="2" />
<ItemDef OID="ID.AEENDAY" SASFieldName="AEENDAY"
Name="Stop Day - Enter Two Digits 01-31" DataType="integer" Length="2" />
<ItemDef OID="ID.AEENYR" SASFieldName="AEENYR"
Name="Stop Year - Enter Four Digit Year" DataType="integer" Length="4" />
<ItemDef OID="ID.AEENDT" SASFieldName="AEENDT"
Name="Derived Stop Date" DataType="date"/>
<ItemDef OID="ID.AESEV" SASFieldName="AESEV"
Name="Severity” DataType="text" Length="1">
<CodeListRef CodeListOID="CL.$AESEV" />
</ItemDef>
<ItemDef OID="ID.AEREL" SASFieldName="AEREL"
Name="Relationship to study drug" DataType="text" Length="1">
<CodeListRef CodeListOID="CL.$AEREL" />
</ItemDef>
Here is a partial listing
of the data in the same sample ODM XML file:
<ClinicalData StudyOID="Study.OID" MetaDataVersionOID="MetaDataVersion.OID.1">
<SubjectData SubjectKey="S001P011" TransactionType="Insert">
<StudyEventData StudyEventOID="StudyEventDefs.OID.6.AdverseEvent"
StudyEventRepeatKey="1">
<FormData FormOID="FormDefs.OID.AE" FormRepeatKey="1">
<ItemGroupData ItemGroupOID="ItemGroupDefs.OID.AE"
ItemGroupRepeatKey="1">
<ItemData ItemOID="ID.TAREA" Value="ONC" />
<ItemData ItemOID="ID.PNO" Value="143-02" />
<ItemData ItemOID="ID.SCTRY" Value="USA" />
<ItemData ItemOID="ID.F_STATUS" Value="V" />
<ItemData ItemOID="ID.LINE_NO" Value="1" />
<ItemData ItemOID="ID.AETERM" Value="HEADACHE" />
<ItemData ItemOID="ID.AESTMON" Value="06" />
<ItemData ItemOID="ID.AESTDAY" Value="10" />
<ItemData ItemOID="ID.AESTYR" Value="1999" />
<ItemData ItemOID="ID.AESTDT" Value="1999-06-10" />
<ItemData ItemOID="ID.AEENMON" Value="06" />
<ItemData ItemOID="ID.AEENDAY" Value="14" />
<ItemData ItemOID="ID.AEENYR" Value="1999" />
<ItemData ItemOID="ID.AEENDT" Value="1999-06-14" />
<ItemData ItemOID="ID.AESEV" Value="1" />
<ItemData ItemOID="ID.AEREL" Value="0" />
<ItemData ItemOID="ID.AEOUT" Value="1" />
<ItemData ItemOID="ID.AEACTTRT" Value="0" />
<ItemData ItemOID="ID.AECONTRT" Value="1" />
</ItemGroupData>
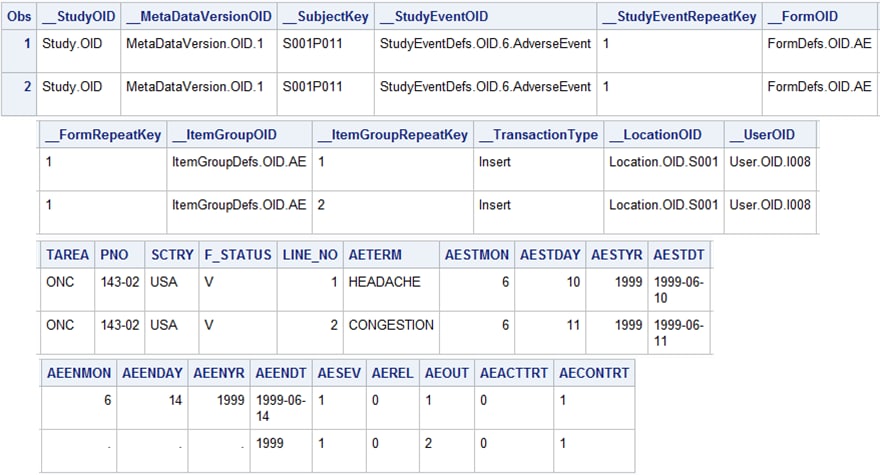
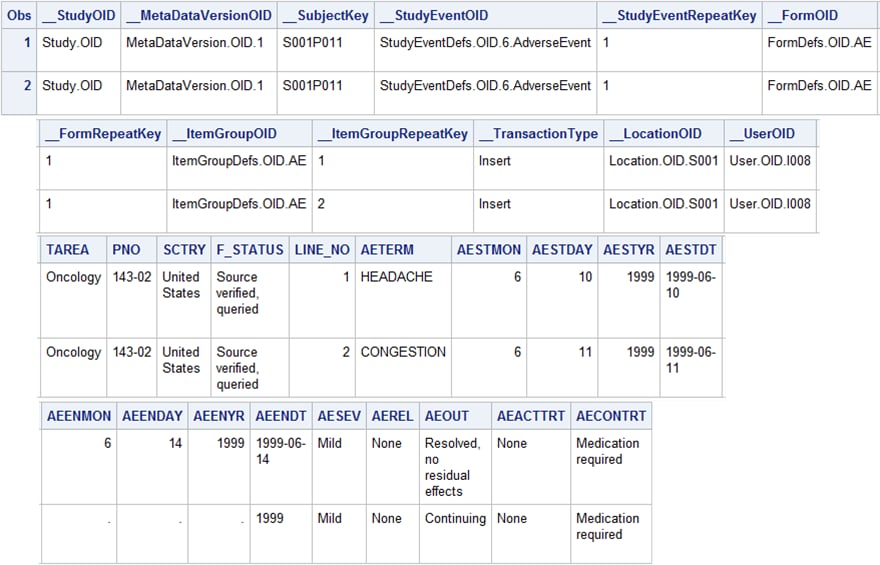
The
%ODM_EXTRACTDOMAINDATA macro creates the data set shown in AE SAS Data Set (Unformatted) Created by the %ODM_EXTRACTDOMAINDATA Macroand AE SAS Data Set (Formatted) Created by the %ODM_EXTRACTDOMAINDATA Macro. The first 12 columns in this data set are the data set
keys. The macro parameter _cstODMMinimumKeyset determines whether
these keys are part of the extracted data set.
AE SAS Data Set (Unformatted) Created by the %ODM_EXTRACTDOMAINDATA
Macro
AE SAS Data Set (Formatted) Created by the %ODM_EXTRACTDOMAINDATA
Macro
The %ODM_EXTRACTDOMAINDATA
macro has this signature:
%macro odm_extractdomaindata( _cstSourceMetadata=, _cstSourceData=, _cstIsReferenceData=No, _cstSelectAttribute=Name, _cstSelectAttributeValue=, _cstLang=en, _cstMaxLabelLength=256, _cstAttachFormats=Yes, _cstODMMinimumKeyset=No, _cstOutputLibrary=, _cstOutputDS= );
Here are the parameters:
-
_cstSourceMetadata and _cstSourceData contain the SAS libref for the SAS ODM metadata representation data.If this is not specified, the macro looks for type=sourcedata in SASReferences. If this is not provided, the data set source is assumed to be in the SAS Work library.
-
_cstIsReferenceData indicates whether the data to extract is reference data or clinical data. Examples of reference data are laboratory reference ranges or trial design data.
-
_cstSelectAttribute contains the ItemGroup attribute that identifies which ItemGroup to extract. Valid values are OID, Name, SASDatasetName, and Domain.
-
_cstSelectAttributeValue contains the value of the attribute defined by _cstSelectAttribute that identifies the ItemGroup to extract.
-
_cstLang specifies a language identifier for the language tag attribute (xml:lang) in the ODM TranslatedText elements.
-
_cstMaxLabelLength determines the maximum value of labels to be created.If this is not provided, 256 is assumed. Formats are attached to the data set variables in case the parameter _cstAttachFormats has a value of ‘Yes’.
-
_cstODMMinimumKeyset determines the creation of data set keys. If this is not provided, ‘No’ is assumed.
-
_cstOutputLibrary defines the SAS library where the extracted data sets are written.If this is not specified, the macro looks for type=targetdata in SASReferences. If this is not provided, the data sets are written to the SAS Work library.
-
_cstOutputDS contains the name of the extracted data set.If this is an invalid SAS data set name, an error is generated. If the data set name is not provided, the macro looks for type=targetdata in SASReferences.
Two sample driver programs
for ODM 1.3.0 are provided with the SAS Clinical Standards Toolkit
to demonstrate the use of the %ODM_EXTRACTDOMAINDATA macro:
sample study library directory/cdisc-odm-1.3.0-1.7/programs/extract_domaindata_all.sassample study library directory/cdisc-odm-1.3.0-1.7/programs/extract_domaindata.sasTwo sample driver programs
for ODM 1.3.1 are provided with the SAS Clinical Standards Toolkit
to demonstrate the use of the %ODM_EXTRACTDOMAINDATA macro:
sample study library directory/cdisc-odm-1.3.1-1.7/programs/extract_domaindata_all.sassample study library directory/cdisc-odm-1.3.1-1.7/programs/extract_domaindata.sasThe extract_domaindata_all.sas
sample driver programs demonstrate how all data sets can be extracted
at once. The following shows a code fragment:
filename incCode CATALOG "work._cstCode.domains.source" lrecl=255;
data _null_;
set srcdata.itemgroupdefs(keep=OID Name IsReferenceData SASDatasetName Domain);
file incCode;
length macrocall $400 _cstOutputName $100;
_cstOutputName=SASDatasetName;
* If we have to use the Name, Only use letters and digits;
if missing(_cstOutputName) then _cstOutputName=cats(compress(Name, 'adk'));
* If first character a digit, prepend an underscore;
if anydigit(_cstOutputName)=1 then _cstOutputName=cats('_', _cstOutputName);
* Cut long names;
if length(_cstOutputName) > 32 then _cstOutputName=substr(_cstOutputName, 1, 32);
macrocall=cats('%odm_extractdomaindata(_cstSelectAttribute=OID',
', _cstSelectAttributeValue=', OID,
', _cstIsReferenceData=', IsReferenceData,
', _cstMaxLabelLength=256',
', _cstAttachFormats=Yes',
', _cstODMMinimumKeyset=No',
', _cstLang=en',
', _cstOutputDS=', _cstOutputName, ');');
put macrocall;
run;
%include incCode;
filename incCode clear;
Reading CDISC ODM Controlled Terminology XML Files: %CT_READ Macro
To read an ODM controlled
terminology XML file as published quarterly by NCI, a specialized
macro named %CT_READ is available in the CDISC controlled terminology
1.0 standards macros folder. This folder is located here:
global standards library directory/standards/cdisc-ct-1.0-1.7/macrosThis macro is referenced
from the create_sasct_fromxml.sas driver program. For
more information, see Sample Driver Program: create_sasct_fromxml.sas .
File references and
other metadata that are required by the macro are set as global macro
variable values. These global macro variable values are set through
the framework initialization properties and the CDISC controlled terminology
1.0 initialization properties. Throughout the processing of the %CT_READ
macro, the Results data set contains all framework-specific messages
and CDISC controlled terminology 1.0-specific messages that were generated
during run time.
Based on file references
defined in the SASReferences data set, the %CT_READ macro accesses
the ODM controlled terminology XML file.
The following display
shows a partial listing of a sample ODM controlled terminology XML
file:
Partial Listing of a Sample ODM Controlled Terminology XML
File

After the %CT_READ macro
confirms that the ODM controlled terminology XML file exists, a call
is made to the SAS DATA step component JavaObj. JavaObj processing
converts the ODM controlled terminology XML file into a cubeXML file
through transformations using XSL files and processes.
The cubeXML file is
created in the SAS Work library. The name of the cubeXML file is _cubnnnn.xml,
where nnnn is a randomly
generated number.
The cubeXML file is
accessed using the SAS XML LIBNAME engine and XMLMap processing. A
default XMLMap file is stored in the sample CDISC controlled terminology
1.0 study folder hierarchy (
referencexml/odm.map).
An odm.map file is required to process the cubeXML file. If it does
not exist, the %CT_READ macro attempts to create one using the CDISC
controlled terminology reference metadata.
Here is a partial listing
of the odm.map file.
<?xml version="1.0" encoding="UTF-8"?>
<SXLEMAP name="CT100" version="1.2">
<TABLE name="CodeLists">
<TABLE-PATH syntax="XPath">/LIBRARY/CodeLists</TABLE-PATH>
<TABLE-DESCRIPTION>Codelist metadata</TABLE-DESCRIPTION>
<COLUMN name="OID">
<PATH syntax="Xpath">/LIBRARY/CodeLists/OID</PATH>
<TYPE>character</TYPE>
<DATATYPE>character</DATATYPE>
<DESCRIPTION>Unique identifier for this codelist</DESCRIPTION>
<LENGTH>128</LENGTH>
</COLUMN>
<COLUMN name="Name">
<PATH syntax="Xpath">/LIBRARY/CodeLists/Name</PATH>
<TYPE>character</TYPE>
<DATATYPE>character</DATATYPE>
<DESCRIPTION>CodeList name</DESCRIPTION>
<LENGTH>128</LENGTH>
</COLUMN>
<COLUMN name="DataType">
<PATH syntax="Xpath">/LIBRARY/CodeLists/DataType</PATH>
<TYPE>character</TYPE>
<DATATYPE>character</DATATYPE>
<DESCRIPTION>CodeList item value data type (integer | float | text | string)</DESCRIPTION>
<LENGTH>7</LENGTH>
</COLUMN>
<COLUMN name="SASFormatName">
<PATH syntax="Xpath">/LIBRARY/CodeLists/SASFormatName</PATH>
<TYPE>character</TYPE>
<DATATYPE>character</DATATYPE>
<DESCRIPTION>SAS format name</DESCRIPTION>
<LENGTH>8</LENGTH>
</COLUMN>
<COLUMN name="ExtCodeID">
<PATH syntax="Xpath">/LIBRARY/CodeLists/ExtCodeID</PATH>
<TYPE>character</TYPE>
<DATATYPE>character</DATATYPE>
<DESCRIPTION>Unique numeric code randomly generated by NCI Thesaurus (NCIt)</DESCRIPTION>
<LENGTH>64</LENGTH>
</COLUMN>
<COLUMN name="CodeListExtensible">
<PATH syntax="Xpath">/LIBRARY/CodeLists/CodeListExtensible</PATH>
<TYPE>character</TYPE>
<DATATYPE>character</DATATYPE>
<DESCRIPTION>Defines if controlled terms may be added to the codelist (Yes | No)</DESCRIPTION>
<LENGTH>3</LENGTH>
</COLUMN>
<COLUMN name="CDISCSubmissionValue">
<PATH syntax="Xpath">/LIBRARY/CodeLists/CDISCSubmissionValue</PATH>
<TYPE>character</TYPE>
<DATATYPE>character</DATATYPE>
<DESCRIPTION>Specific value expected for submissions</DESCRIPTION>
<LENGTH>512</LENGTH>
</COLUMN>When the cubeXML file
is processed, each of the 15 data sets (such as CodeLists) that are
included in the SAS representation of the CDISC controlled terminology
model is derived. One input parameter can be specified in the call
to the %CT_READ macro. The parameter offers the option to create source
metadata files.
Note: For more information about
the %CT_READ macro, see the SAS Clinical Standards Toolkit: Macro API Documentation.
By default, if a %CT_READ
macro call is made with null parameters, source metadata is derived.
The target location of the derived metadata files is defined in the
SASReferences data set.
Sample Driver Program: create_sasct_fromxml.sas
Overview
Each primary SAS Clinical
Standards Toolkit task, such as reading CDISC ODM controlled terminology
XML files, is guided by a sample driver program that is provided with
the SAS Clinical Standards Toolkit. For reading ODM controlled terminology
XML files, this driver program is create_sasct_fromxml.sas.
This driver program
is located here:
sample study library directory/cdisc-ct-1.0-1.7/programsThe SASReferences Data Set
As part of each SAS
Clinical Standards Toolkit process setup, a valid SASReferences data
set is required. The SASReferences data set references the input files
that are needed (such as the ODM controlled terminology XML file),
the librefs and filenames to use, and the names and locations of the
data sets to create. The SASReferences data set can be modified to
point to study-specific files.
For more information,
see SASReferences File.
In the SASReferences
data set, there are two input file references and five output data
set references that are key to the successful completion of the driver
program. Key Components of the SASReferences Data Set for the create_sasct_fromxml.sas Driver
Program lists these
files and data sets. In the sample create_sasct_fromxml.sas
driver program, these values are set for &studyRootPath and &studyOutputPath:
&studyRootPath=sample study library directory/cdisc-ct-1.0-1.7&studyOutputPath=sample study library directory/cdisc-ct-1.0-1.7|
Metadata Type
|
SAS LIBNAME or Fileref
to Use
|
Reference Type
|
Path
|
Name of File
|
|---|---|---|---|---|
|
Input
|
||||
|
externalxml
|
crtxml
|
fileref
|
&studyRootPath/sourcexml/sdtm/201212
|
sdtm_terminology.xml
|
|
referencexml
|
ctmap
|
fileref
|
&studyRootPath/referencexml
|
ct-1.0.0.map
|
|
Output
|
||||
|
sourcedata
|
srcdata
|
libref
|
&studyOutputPath/data/sdtm/201212
|
*.*
|
|
results
|
results
|
libref
|
&studyOutputPath/results
|
read_results_sdtm_2012.sas7bdat
|
Process Inputs
The externalxml type
refers to the ODM controlled terminology XML file to read. The filename
reference crtxml is defined in the SASReferences data set. This filename
reference is used in the submitted SAS code when referring to the
ODM controlled terminology XML file.
The referencexml type
refers to the SAS map file that is used to generate the SAS data sets
that represent the ODM file metadata and content. The filename reference
ctmap is defined in the SASReferences data set. This filename reference
is used in the submitted SAS code when referring to the SAS map file.
If a path and filename for the map file are not specified, a temporary
map file is created as part of the %CT_READ macro processing.
Process Outputs
When the driver program
finishes running, the read_results_sdtm_201212 data set is created
in the Results library. This data set contains informational messages,
warnings, and error messages that were generated by the driver program.
The following display
shows an example of the contents of a Results data set that was created
while reading the sample ODM controlled terminology XML file as released
by NCI that was provided with the SAS Clinical Standards Toolkit:
Example of a Partial Results Data Set Created by the create_sasct_fromxml.sas
Driver Program
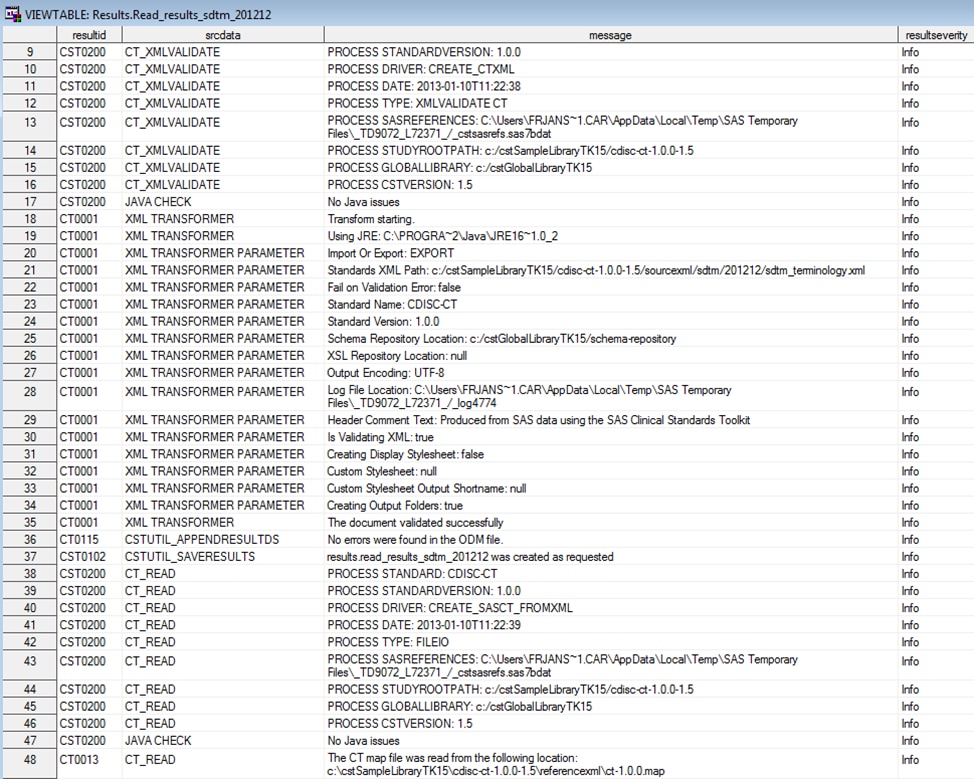
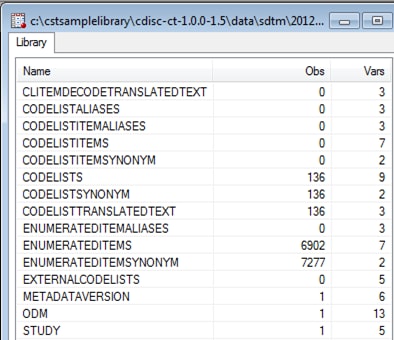
The Srcdata library
contains the SAS data sets that represent the ODM controlled terminology
XML file metadata and content. By default, the %CT_READ macro creates
15 unique data sets in the SAS Clinical Standards Toolkit. Some of
these data sets might be empty if no associated content was derived
from the ODM controlled terminology XML file. There is a one-to-one
correspondence between the tables listed in the Srcdata library and
the tables contained in the source_tables metadata file in the Srcmeta
library.
Example of Partial Srcdata Library Derived from the %CT_READ
Macro
Creating a Format Catalog and a Controlled Terminology Data Set from the SAS Representation of a CDISC ODM Controlled Terminology XML File: %CT_CREATEFORMATS Macro
To use the NCI CDISC
controlled terminology in a SAS Clinical Standards Toolkit process,
the SAS data sets created by the %CT_READ macro must be converted
to a SAS format catalog. To enable SAS Clinical Data Integration to
import controlled terminology, the SAS data set representation created
by the %CT_READ macro must be combined into one SAS data set.
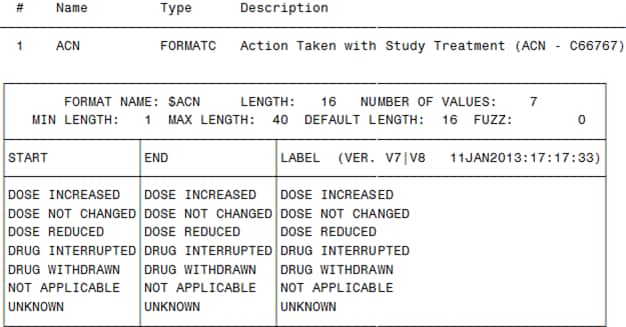
The following display
shows an example of controlled terminology in ODM XML (the Action
Taken with Study Treatment codelist):
Example of Controlled Terminology in ODM XML

The following display
shows the data set created by the %CT_CREATEFORMATS macro:
Partial cterms SAS Data Set Created by the %CT_CREATEFORMATS
Macro

The following display
shows that the %CT_CREATEFORMATS macro uses the data set to create
the $ACN SAS format:
$ACN SAS Format Created by the %CT_CREATEFORMATS Macro
%macro ct_createformats(
_cstLang=en, /* Language tag in TranslatedText to use */
_cstCreateCatalog=1, /* Create format catalog */
_cstKillCatFirst=0, /* Empty catalog first */
_cstUseExpression=, /* Expression to create the SAS format name */
_cstAppendChar=F, /* Letter to append in case SAS format name
ends with digit */
_cstDeleteEmptyColumns=1, /* Delete columns in output data set that are
completely missing */
_cstTrimCharacterData=1 /* Truncate character data in output data set
to the minimum value needed. */
);
The %CT_CREATEFORMATS
macro attempts to map the CodeList/nciodm:CDISCSubmissionValue in
the codelist variable to the fmtname variable. The fmtname variable
value must contain a valid SAS format name. The %CT_CREATEFORMATS
macro uses the following steps to create a valid SAS format name:
-
Apply a user-defined expression to create the fmtname variable.
-
If the value of fmtname is empty, use the CodeList/SASFormatName attribute (typically empty in NCI EVS ODM XML files).
-
If the value of fmtname is empty, use the CodeList/nciodm:CDISCSubmissionValue value in the codelist variable.
-
If the value of fmtname ends with a digit, add the character specified by the _cstAppendChar macro parameter (default=F).
After these steps, the
value of the fmtname variable is validated against the following regular
expression:
'm/^(?=.{1,32}$)([\$a-zA-Z_][a-zA-Z0-9_]*[a-zA-Z_])$/'If the value of the
fmtname variable fails validation, the fmtname variable value does
not contain a valid SAS format name. The value is set to missing.
Then, the codelist is not used to create a SAS format.
Two sample driver programs
are provided with the SAS Clinical Standards Toolkit to demonstrate
the use of the %CT_CREATEFORMATS macro:
sample study library directory/cdisc-ct-1.0-1.7/programs/create_ctformats.sassample study library directory/cdisc-ct-1.0-1.7/programs/create_ctformats_qs.sasBoth of these sample
driver programs demonstrate how CDISCSubmissionValue can be mapped
to a valid SAS format name.
Reading CDISC CRT-DDS 1.0 or Define-XML 2.0 define.xml Files: %CRTDDS_READ and %DEFINE_READ Macros
The process for reading
CDISC CRT-DDS 1.0 and CDISC Define-XML 2.0 define.xml files is similar
to reading CDISC ODM XML files.
Note: This section demonstrates
reading CDISC CRT-DDS 1.0 define.xml files as an example. The CDISC
Define-XML 2.0 process is similar, but uses the define_read macro
instead of the crtdds_read macro.
The SAS Clinical Standards
Toolkit supports reading a define.xml file and translating the file
metadata into a SAS representation of the CDISC CRT-DDS model. To
read the define.xml file, a specialized macro named %CRTDDS_READ is
available in the CRT-DDS 1.0 standards macros folder. This folder
is located in
global standards library directory/standards/cdisc-crtdds-1.0-1.7/macros.
This macro is referenced
from the create_sascrtdds_fromxml.sas driver program. There are no
input parameters in the call to the %CRTDDS_READ macro.
File references and
other metadata that are required by the macro are set as global macro
variables. These global macro variables are set through the framework
initialization properties and the CDISC CRT-DDS 1.0 initialization
properties. Throughout the processing of the %CRTDDS_READ macro, the
Results data set contains all framework-specific messages and CRT-DDS
1.0-specific messages that were generated during run time.
Based on file references
defined in the SASReferences data set, the %CRTDDS_READ macro accesses
the define.xml file.
Here is a partial listing
of a sample define.xml file.
<ODM xmlns:xlink="http://www.w3.org/1999/xlink"
xmlns:def="http://www.cdisc.org/ns/def/v1.0"
xmlns="http://www.cdisc.org/ns/odm/v1.2" FileOID="1"
CreationDateTime="2011-07-13T17:15:43-04:00"
AsOfDateTime="2011-07-13T17:12:42"
Description="define1" FileType="Snapshot" Id="define1"
ODMVersion="1.0">
<Study OID="1">
<GlobalVariables>
<StudyName>study1</StudyName>
<StudyDescription>first study</StudyDescription>
<ProtocolName>Protocol abc</ProtocolName>
</GlobalVariables>
<MetaDataVersion OID="1" Name="CDISC-SDTM 3.1.2"
Description="CDISC-SDTM 3.1.2"
def:DefineVersion="1.0.0"
def:StandardName="CDISC SDTM"
def:StandardVersion="3.1.2">
<ItemGroupDef
OID="AE1" Name="AE" Repeating="Yes"
IsReferenceData="No"
SASDatasetName="AE" Domain="AE"
Purpose="Tabulation" def:Label="Adverse Events"
def:Class="Events"
def:Structure="One record per adverse event per subject"
def:DomainKeys="STUDYID USUBJID AEDECOD AESTDTC"
def:ArchiveLocationID="AE1">
<ItemRef ItemOID="COL1" Mandatory="Yes"
OrderNumber="1" KeySequence="1" Role="Identifier"/>
<ItemRef ItemOID="COL2" Mandatory="Yes"
OrderNumber="2" Role="Identifier"/>
<ItemRef ItemOID="COL3" Mandatory="Yes"
OrderNumber="3" KeySequence="2" Role="Identifier"/>
<ItemRef ItemOID="COL4" Mandatory="Yes"
OrderNumber="4" Role="Identifier"/>
<ItemRef ItemOID="COL5" Mandatory="No"
OrderNumber="5" Role="Identifier"/>
<ItemRef ItemOID="COL6" Mandatory="No"
OrderNumber="6" Role="Identifier"/>
<ItemRef ItemOID="COL7" Mandatory="No"
OrderNumber="7" Role="Identifier"/>
After the %CRTDDS_READ
macro confirms that the define.xml file exists, a call is made to
the SAS DATA step component JavaObj. JavaObj processing converts the
define.xml file into a cubeXML file through transformations using
XSL files and processes.
The cubeXML file is
created in the Work library. The name of the cubeXML file is _cubnnnn.xml
, where nnnn is a randomly
generated number.
The cubeXML file is
accessed using the SAS XML LIBNAME engine and XMLMap processing. A
default XMLMap file is stored in the sample CRT-DDS 1.0 study folder
hierarchy (
referencexml/define.map).
The define.map file is required to process the cubeXML file. If it
does not exist, the crtdds_read attempts to create one using the CRT-DDS
reference metadata.
Here is a partial listing
of the define.map file.
<?xml version="1.0" encoding="windows-1252"?>
<SXLEMAP version="1.2">
<TABLE name="AnnotatedCRFs">
<TABLE-PATH syntax="XPath">/LIBRARY/AnnotatedCRFs</TABLE-PATH>
<TABLE-DESCRIPTION>Annotated CRF metadata</TABLE-DESCRIPTION>
<COLUMN name="DocumentRef">
<PATH syntax="Xpath">/LIBRARY/AnnotatedCRFs/DocumentRef</PATH>
<TYPE>character</TYPE>
<DATATYPE>character</DATATYPE>
<DESCRIPTION>The referenced Annotated CRF document</DESCRIPTION>
<LENGTH>2000</LENGTH>
</COLUMN>
<COLUMN name="leafID">
<PATH syntax="Xpath">/LIBRARY/AnnotatedCRFs/leafID</PATH>
<TYPE>character</TYPE>
<DATATYPE>character</DATATYPE>
<DESCRIPTION>The unique ID of the referenced Annotated CRF</DESCRIPTION>
<LENGTH>128</LENGTH>
</COLUMN>
<COLUMN name="FK_MetaDataVersion">
<PATH syntax="Xpath">/LIBRARY/AnnotatedCRFs/FK_MetaDataVersion</PATH>
<TYPE>character</TYPE>
<DATATYPE>character</DATATYPE>
<DESCRIPTION>Foreign key: MetaDataVersion.OID</DESCRIPTION>
<LENGTH>128</LENGTH>
</COLUMN>
</TABLE>
Processing of the cubeXML
file results in the derivation of the data sets (such as ItemDefs)
currently included in the SAS representation of the CDISC CRT-DDS
model.
The final step in the
%CRTDDS_READ macro is the derivation of table and column metadata
that describe the data sets in the SAS representation of the define.xml
file. At this point, the %CRTDDS_READ macro is ready to create the
source_tables and source_columns data sets. The tables in the source_tables
data set are created and copied to the output library as defined in
the SASReferences data set.
Sample Driver Program: create_sascrtdds_fromxml.sas and create_sasdefine_fromxml.sas
Overview
Each primary SAS Clinical
Standards Toolkit task, such as reading CDISC CRT-DDS 1.0 or CDISC
Define-XML 2.0 XML files, is guided by a sample driver program that
is provided with the SAS Clinical Standards Toolkit.
Note: CDISC CRT-DDS 1.0 is discussed
in this section. The process is similar for CDISC Define-XML 2.0.
The create_sascrtdds_fromxml.sas
driver program is used to read define.xml files.
The driver program is
located here:
sample study library directory/cdisc-crtdds-1.0–1.7/programsThe SASReferences Data Set
As a part of
each SAS Clinical Standards Toolkit process setup, a valid SASReferences
data set is required. It references the input files that are needed,
the librefs and filenames to use, and the names and locations of data
sets to be created by the process. It can be modified to point to
study-specific files. For
an explanation of the SASReferences data set, see SASReferences File.
In the SASReferences
data set, there are two input file references and four output data
set references that are key to the successful completion of the driver
program. Key Components of the SASReferences Data Set for the create_sascrtdds_fromxml.sas
Driver Program lists these
files and data sets, and they are discussed in separate sections. In the sample create_sascrtdds_fromxml.sas driver program,
these values are set for &studyRootPath and &studyOutputPath:
&studyRootPath=&_cstSRoot/cdisc-crtdds-1.0-&_cstVersion&studyOutputPath=&_cstSRoot/cdisc-crtdds-1.0-&_cstVersion|
Metadata Type
|
SAS LIBNAME or Fileref
to Use
|
Reference Type
|
Path
|
Name of File
|
|---|---|---|---|---|
|
Input
|
||||
|
externalxml
|
crtxml
|
fileref
|
&studyRootPath/sourcexml
|
define.xml
|
|
referencexml
|
crtmap
|
fileref
|
&studyRootPath/referencexml
|
define.map
|
|
Output
|
||||
|
sourcedata
|
srcdata
|
libref
|
&studyOutputPath/deriveddata
|
*.*
|
|
sourcemetadata
|
srcmeta
|
libref
|
&studyOutputPath/derivedmetadata
|
source_tables.sas7bdat
|
|
sourcemetadata
|
srcmeta
|
libref
|
&studyOutputPath/derivedmetadata
|
source_columns.sas7bdat
|
|
sourcemetadata
|
srcmeta
|
libref
|
&studyOutputPath/derivedmetadata
|
source_study.sas7bdat
|
|
results
|
results
|
libref
|
&studyOutputPath/results
|
read_results.sas7bdat
|
Process Inputs
The externalxml type
refers to the define.xml file to read. The filename reference crtxml
is defined in the SASReferences data set. This filename reference
is used in the submitted SAS code when referring to the define.xml
file.
The referencexml type
refers to the SAS map file that is used to generate the SAS data sets
that represent the define.xml file metadata and content. The filename
reference crtmap is defined in the SASReferences data set. This filename
is used in the submitted SAS code when referring to the SAS map file.
If a path and filename for the map file are not specified, a temporary
map file is created as part of the crtdds_read processing.
Process Outputs
The sourcedata type
is the library where the metadata files are created. These metadata
files are the data sets that comprise the CRT-DDS information.
The sourcemetadata type
refers to two data sets that are created from the cubeXML file, source_tables,
and source_columns. Both data sets are stored in the same library.
The source_tables data set contains metadata about each table that
is derived from the CRT-DDS macro. The source_columns data set contains
similar metadata but it is at the column level. Both of the data sets
are written to the Srcmeta library. The sourcemetadata type refers
to a data set source_study. The source_study data set is created in
the Srcmeta library and contains study metadata.
The results type refers
to the Results data set that contains information from running the
CRT-DDS macro. This information is written to the read_results data
set in the Results library.
Process Results
When the driver program
finishes running, the read_results data set is created in the Results
library. This data set contains informational, warning, and error
messages that were generated by the driver program.
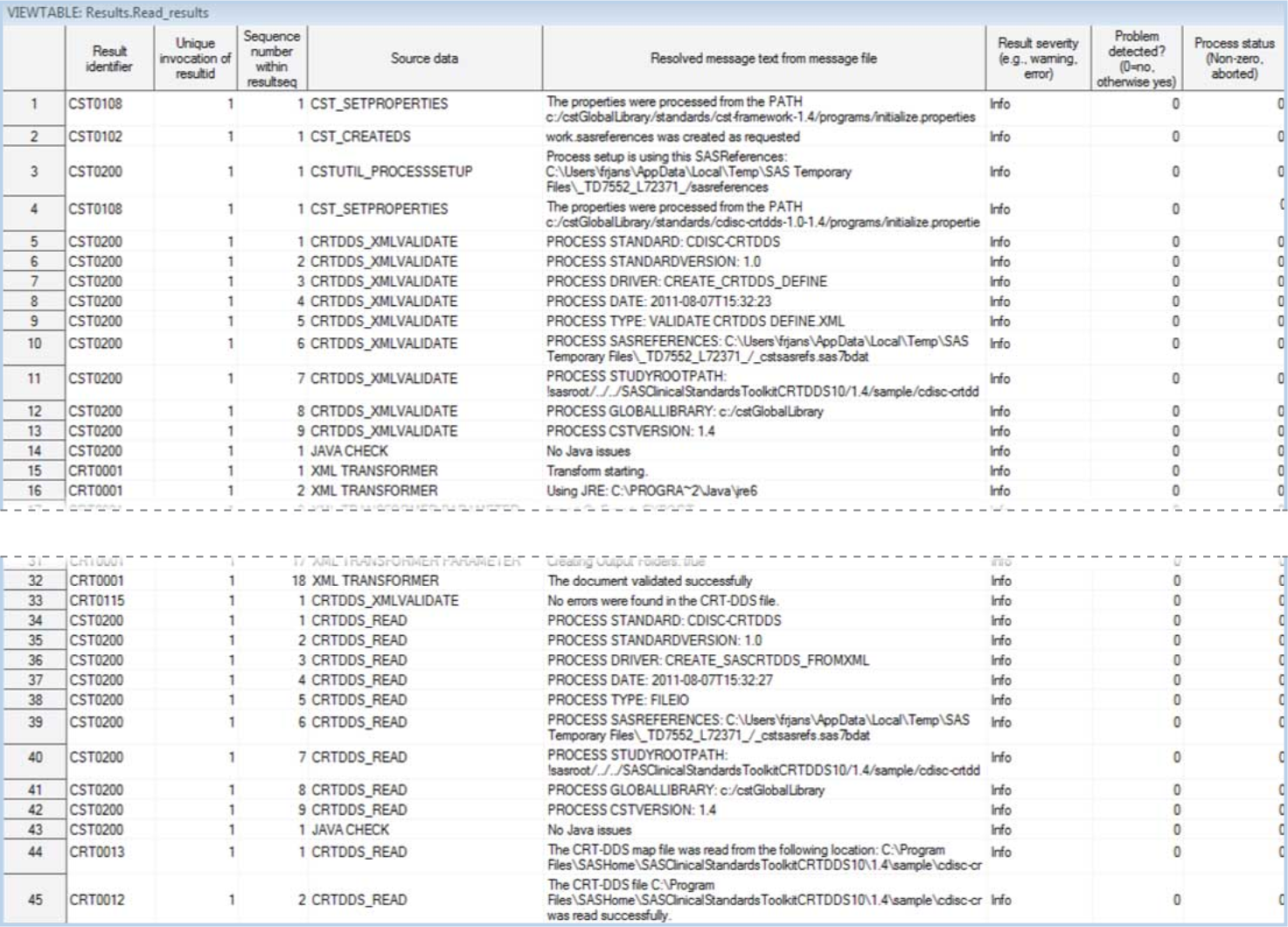
The following display
shows an example of the contents of a Results data set in the CRT-DDS
sample study:
Example of a Partial Results Data Set Created by the create_sascrtdds_fromxml.sas
Driver Program
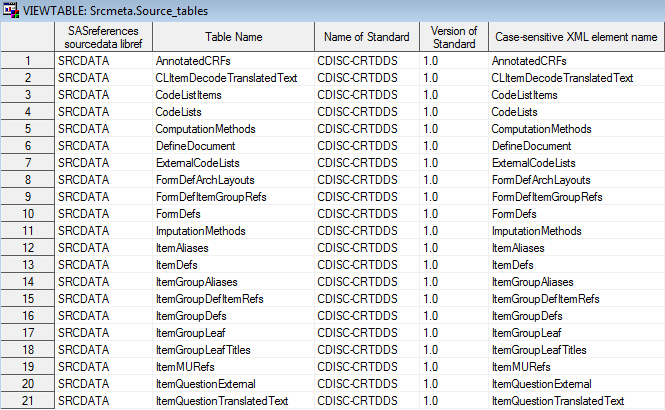
The %CRTDDS_READ macro
creates the source_tables and source_columns data sets in the Srcmeta
library. These data sets contain the table and column metadata for
the SAS representation of CRT-DDS that is derived from the define.xml
file. The Srcmeta library corresponds to the location specified in
SASReferences (
&studyOutputPath/derivedmetadata).
Example of Partial Source_Tables Data Set Derived from the
%CRTDDS_READ Macro
Example of Partial Source_Columns Data Set Derived from the
%CRTDDS_READ Macro
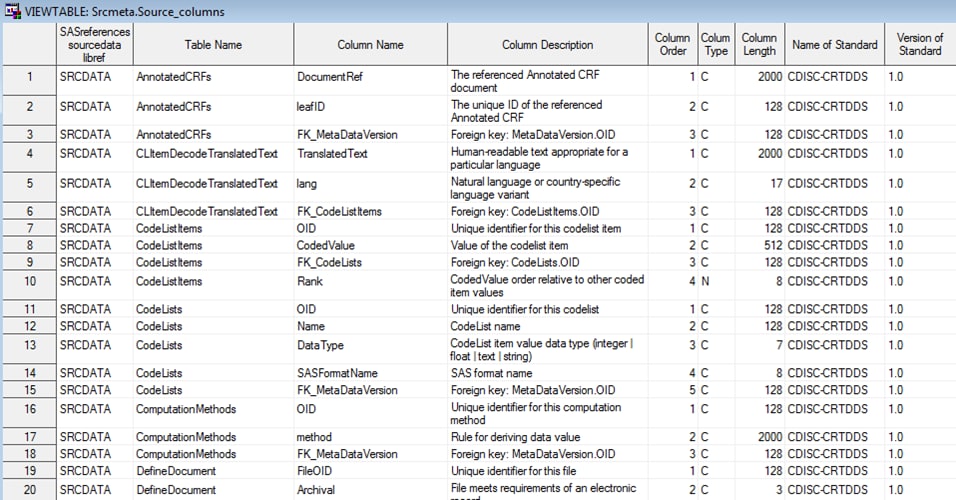
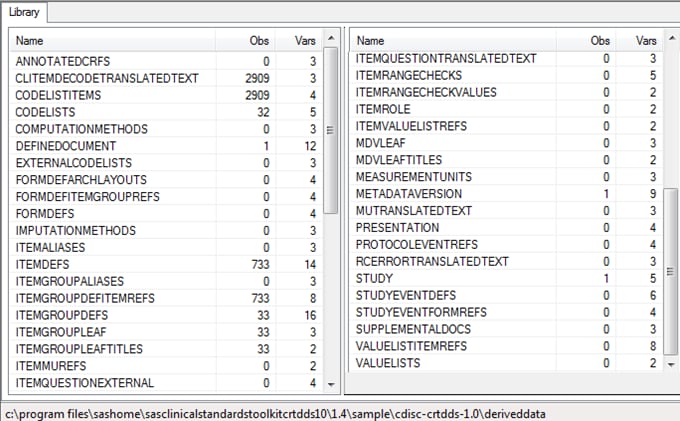
The Srcdata library
contains the driver program-generated tables that comprise the SAS
representation of the CRT-DDS model. There is a one-to-one correspondence
between the tables listed in the Srcdata library and the tables contained
in the source_tables metadata file in the Srcmeta library. The Srcdata
library corresponds to the location specified in SASReferences (&studyOutputPath/deriveddata).
Example of Partial Srcdata Library Derived from the %CRTDDS_READ
Macro
When running the driver
programs against non-sample data, you must populate the SASReferences
data set in the driver program with the proper values. For
an explanation of the SASReferences data set, see SASReferences File.
Copyright © SAS Institute Inc. All Rights Reserved.