Writing XML Files
Overview
Support of CDISC XML-based
standards, such as CDISC CRT-DDS 1.0, CDISC Define-XML 2.0, and CDISC
ODM, includes the ability to render these files in SAS data set format
and the ability to create model-specific XML files from a SAS data
set representation of those standards.
In the SAS Clinical
Standards Toolkit, you can create a CDISC CRT-DDS 1.0 define.xml file
or CDISC Define-XML 2.0 file (including Analysis Results Metadata
1.0) that references a CDISC SDTM study, a SEND study, or a CDISC
ADaM study. You can also create a CDISC ODM 1.3.0 XML file or a CDISC
ODM 1.3.1 file.
The next section outlines
the basic workflow for the creation of model-specific XML files.
Basic Workflow
Here is the basic workflow
for writing XML files:
-
Build the SAS representation of a given XML-based standard by referencing an existing set of data and metadata about a clinical study, or by creating data and metadata about a new clinical study in the standard-specific SAS format.
-
(Optional) Validate the SAS representation of the XML-based standard (to include foreign key relationships, value conformance to a set of expected values, and so on).
-
Create a standardized intermediate cubeXML file using the data and metadata contained in the SAS representation of the standard.
-
(Build and) reference a set of valid XSL style sheets for each target data set (such as ItemDefs.xsl).
-
Use the SAS DATA step component JavaObj to read the cubeXML file using the XSL style sheets to create the target standard-specific XML file.
-
(Optional) Validate the structure and syntax of the XML file that was created against an XML schema.
Creating a CDISC CRT-DDS 1.0 define.xml File
There are four key macros
that are provided with the SAS Clinical Standards Toolkit that support
creation of a CDISC CRT-DDS 1.0 define.xml file. The four macros are
listed in the order in which they are executed:
-
The %CRTDDS_SDTMTODEFINE macro creates the 39 tables for the SAS representation of the CRT-DDS files from SDTM metadata. This macro, using SDTM table and column metadata as its source, populates a subset of 19 CRT-DDS data sets.The %CRTDDS_ADAMTODEFINE macro is similar to the %CRTDDS_SDTMTODEFINE macro but uses ADaM table and column metadata.
-
The %CRTDDS_VALIDATE macro submits a set of validation checks based on what is defined in the Validation Control data set to validate the referenced SAS representation of the CRT-DDS files.
-
The %CRTDDS_WRITE macro creates the define.xml file from the SAS representation of the CRT-DDS files.
-
The %CSTUTILXMLVALIDATE macro validates that the XML file is structurally and syntactically correct according to the XML schema for the CRT-DDS 1.0 standard. This macro is important if you customize the define.xml file outside of the workflow. For example, if you edit the define.xml file to add links for annotated CRF pages, this macro validates the syntax.
These macros are called
by driver programs that are responsible for properly setting up each
SAS Clinical Standards Toolkit process to perform a specific SAS Clinical
Standards Toolkit task. Several sample driver programs are provided
with the SAS Clinical Standards Toolkit CDISC CRT-DDS standard related
to the creation of the define.xml file.
Here is the purpose
of each of these driver programs:
-
The create_crtdds_from_sdtm.sas driver program sets up the required metadata and SASReferences data set for the sample study. It runs the %CRTDDS_SDTMTODEFINE macro. It creates the SAS representation of the CRT-DDS data sets from the sample study SDTM data sets.
-
The validate_crtdds_data.sas driver program validates the SAS representation of the CRT-DDS define data sets based on the selected CRT-DDS validation checks. This driver program can be run multiple times until data validation has been reconciled.
-
The create_crtdds_define.sas driver program creates the CDISC CRT-DDS 1.0 define.xml file. It runs the %CRTDDS_WRITE and %CSTUTILXMLVALIDATE macros. This driver program creates and validates the XML syntax for the define.xml file.
These driver programs
are examples that are provided with the SAS Clinical Standards Toolkit.
You can use these driver programs or create your own. The names of
these driver programs are not important. However, the content is important
and demonstrates how the various SAS Clinical Standards Toolkit framework
macros are used to generate the required metadata files.
The driver programs
create a define.xml based on SDTM metadata. Similar programs are provided
with the SAS Clinical Standards Toolkit for the creation of a define.xml
based on ADaM metadata.
Sample Driver Program: create_crtdds_from_sdtm.sas
Overview
The create_crtdds_from_sdtm.sas
driver program sets up the required environment variables and library
references to initiate the %CRTDDS_SDTMTODEFINE macro. This macro
extracts data from the SDTM metadata files. (For
more information about the source_tables and source_columns data sets,
see Source Metadata.) Depending on the available source information, the macro
attempts to convert the information into the 39 tables that represent
the SAS interpretation of the CDISC CRT-DDS 1.0 model. All 39 data
sets are created, but only those data sets with available data are
populated. The other tables contain zero observations.
The following table
lists the parameters for the driver program:
|
Parameter
|
Required
|
Description
|
|---|---|---|
|
_cstOutLib
|
Yes
|
The library reference
(LIBNAME) where the tables are created.
|
|
_cstSourceTables
|
Yes
|
The data set that contains
the SDTM metadata for the domains to include in the CRT-DDS file.
|
|
_cstSourceColumns
|
Yes
|
The data set that contains
the SDTM metadata for the domain columns to include in the CRT-DDS
file.
|
|
_cstSourceStudy
|
Yes
|
The data set that contains
the SDTM metadata for the studies to include in the CRT-DDS file.
|
|
_cstSourceValues
|
No
|
The data set that contains
the SDTM metadata for the Value Level columns to include in the CRT-DDS
file.
|
|
_cstSourceDocuments
|
No
|
The data set that contains
the SDTM metadata for the Document references to include in the CRT-DDS
file.
|
Here is an example of
a call to the %CRTDDS_SDTMTODEFINE macro:
%crtdds_sdtmtodefine( _cstOutLib=srcdata, _cstSourceTables=sampdata.source_tables, _cstSourceColumns=sampdata.source_columns, _cstSourceValues=sampdata.source_values, _cstSourceDocuments=sampdata.source_documents, _cstSourceStudy=sampdata.source_study );
In the example, the
%CRTDDS_SDTMTODEFINE macro writes all of the CRT-DDS 1.0 defined tables
to the Srcdata library.
The create_crtdds_from_sdtm.sas
driver program is provided with the SAS Clinical Standards Toolkit,
and it is ready to run on any of the SDTM sample studies. The driver
program can be run interactively or in batch. To run the driver program
interactively, start a SAS session, and load the driver program into
the SAS editor.
The driver program is
located here:
sample study library directory/cdisc-crtdds-1.0–1.7/programsThe SASReferences Data Set
As a part of
each SAS Clinical Standards Toolkit process setup, a valid SASReferences
data set is required. It references the input files that are needed,
the librefs and filenames to use, and the names and locations of data
sets to be created by the process. It can be modified to point to
study-specific files. For
an explanation of the SASReferences data set, see SASReferences File.
In the SASReferences
data set, there are five input file references and one output data
set reference that are key to the successful completion of the create_crtdds_from_sdtm.sas
driver program. Key Components of the SASReferences Data Set for the create_crtdds_from_sdtm.sas Driver
Program lists these
files and data sets, and they are discussed in separate sections. In the sample create_crtdds_from_sdtm.sas driver program,
these values are set for &studyRootPath and &studyOutputPath:
&studyRootPath=sample study library directory/cdisc-sdtm-3.1.3–1.7/sascstdemodata&studyOutputPath=sample study library directory/cdisc-crtdds-1.0–1.7|
Metadata Type
|
SAS LIBNAME or Fileref
to Use
|
Reference Type
|
Path
|
Name of File
|
|---|---|---|---|---|
|
Input
|
||||
|
sourcemetadata
|
sampdata
|
libref
|
&studyRootPath/metadata
|
source_tables.sas7bdat
|
|
sourcemetadata
|
sampdata
|
libref
|
&studyRootPath/metadata
|
source_columns.sas7bdat
|
|
sourcemetadata
|
sampdata
|
libref
|
&studyRootPath/metadata
|
source_study.sas7bdat
|
|
sourcemetadata
|
sampdata
|
libref
|
&studyRootPath/metadata
|
source_values.sas7bdat
|
|
sourcemetadata
|
sampdata
|
libref
|
&studyRootPath/metadata
|
source_documents.sas7bdat
|
|
Output
|
||||
|
sourcedata
|
srcdata
|
libref
|
&studyOutputPath/data
|
|
Process Inputs
The sourcemetadata type
refers to three data sets that contain the SDTM domain metadata: source_tables,
source_columns, and source_values. These data sets are stored in the
same library.
The sample create_crtdds_from_sdtm.sas
driver program provided with the SAS Clinical Standards Toolkit references
a source CDISC SDTM 3.1.3 study. So, the source_tables data set contains
SDTM 3.1.3 metadata about each standard domain defined in the Study
Data Tabulation Model Implementation Guide: Human Clinical Trials
(Version 3.1.3) and includes any customizations that you
have added. The source_columns data set contains similar metadata
but it is at the column level. The source_values data set contains
Value Level metadata. The source metadata is read from this location:
sample study library directory/cdisc-sdtm-3.1.3–1.7/sascstdemodata/metadataThis location is represented
in the driver program by the sampdata library name.
A source study data
set (source_study) is required by this driver program. The following
table lists the variables that are required in this data set:
|
Variable*
|
Required
|
Description
|
|---|---|---|
|
StudyName
|
Yes
|
The name of the study.
This value is used to populate the srcdata.study.studyname column.
|
|
DefineDocumentName
|
Yes
|
The name of the define
document to create. This value is used to populate the srcdata.definedocument.FileOID.
|
|
SASref
|
Yes
|
The reference that ties
the study name to the corresponding domains that are associated with
this study in the source_tables and source_columns data sets.
|
|
ProtocolName
|
Yes
|
The name of the protocol
for the study. This value is used to populate the srcdata.study.protocolname
column.
|
|
StudyDescription
|
Yes
|
The description of the
study. This value is used to populate the srcdata.study.studydescription
column.
Note: You cannot use commas, semicolons,
or quotation marks in the description.
|
|
Standard
|
Yes
|
The name of the standard
in the SAS Clinical Standards Toolkit. (For example, CDISC-SDTM.)
|
|
StandardVersion
|
Yes
|
The version of the standard
in the SAS Clinical Standards Toolkit. (For example, 3.1.3.)
|
|
FormalStandard
|
Yes
|
The formal name of the
standard as used in CRT-DDS. (For example, CDISC SDTM.)
|
|
FormalStandardVersion
|
Yes
|
The formal version of
the standard as used in CRT-DDS. (For example, 3.1.3.)
|
*All variables are required
to be non-blank.
Only a single study
can be referenced in the source study data set.
Process Outputs
The sourcedata type
is the library where the metadata files are created. These metadata
files are the data sets that comprise the SAS representation of the
CDISC CRT-DDS 1.0 standard. The create_crtdds_from_sdtm.sas driver
program creates 39 data sets. Most of these data sets have zero observations
because there is no default SDTM metadata source. In the SAS Clinical
Standards Toolkit sample study, these data sets are written to the
sample study library directory/cdisc-crtdds-1.0–1.7/data directory.
This location is represented in the driver program by the srcdata
library name.
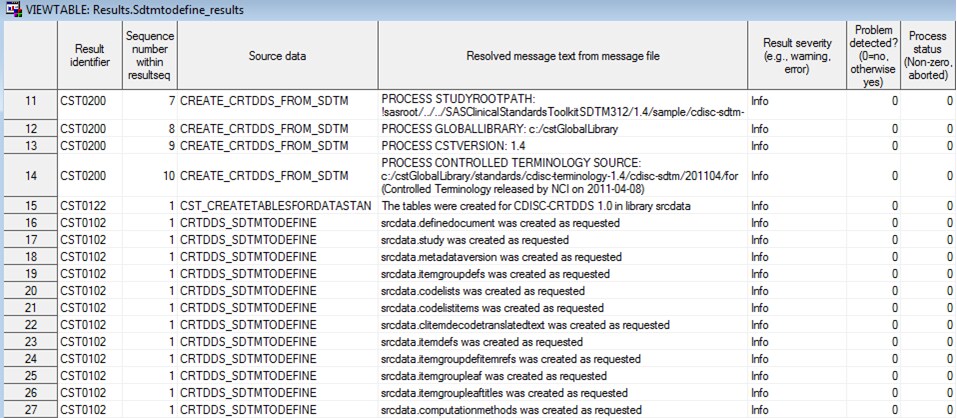
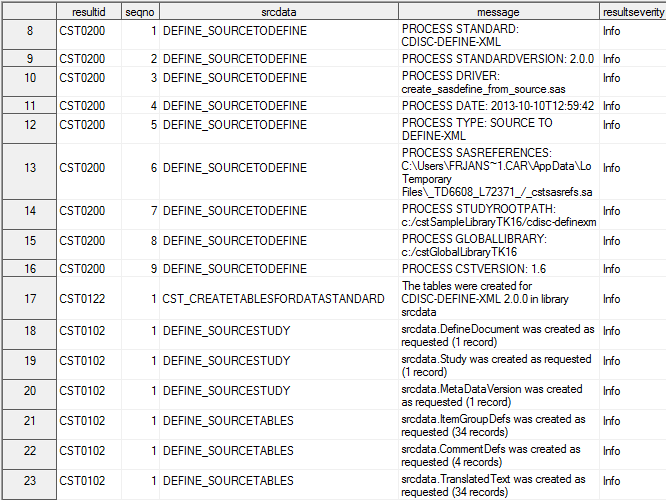
Process Results
When the driver program
finishes running, the sdtmtodefine_results data set is created. This
data set contains informational, warning, and error messages that
were generated by the submitted driver program.
Example of a Partial Results Data Set from CRT-DDS Sample Study
Sample Driver Program: create_crtdds_define.sas
Overview
The create_crtdds_define.sas
driver program sets up the required environment variables and library
references to initiate the %CRTDDS_WRITE macro. This macro reads the
39 data sets that comprise the SAS representation of the CDISC CRT-DDS
1.0 model, and it converts that information to the required define.xml
structure. If source metadata or data are missing, then empty elements
and attributes are not created in the define.xml file. The inputs
and outputs are specified in the SASReferences data set.
Note: For more information about
the %CRTDDS_WRITE macro, see the SAS Clinical Standards Toolkit: Macro API Documentation.
Here is an example of
a call to the %CRTDDS_WRITE macro:
%crtdds_write(_cstCreateDisplayStyleSheet=1,
_cstOutputEncoding=UTF-16,
_cstResultsOverrideDS=&_cstResultsDS);
In this example, a default
style sheet is generated in the same directory as the XML output based
on the information in the SASReferences data set. XML encoding is
set to UTF-16, and process results are written to the default &_cstResultsDS
data set.
Here is the call to
the macro from the sample create_crtdds_define.sas driver program:
%crtdds_write(_cstCreateDisplayStyleSheet=1);
The call creates a display
style sheet and uses default values for the parameters.
The create_crtdds_define.sas
driver program is ready to run on any of the CDISC SDTM sample studies.
The driver program can be run interactively or in batch.
The driver program is
located here:
sample study library directory/cdisc-crtdds-1.0–1.7/programsMultiple tasks can be
executed in any SAS Clinical Standards Toolkit driver program. The
create_crtdds_define.sas driver program calls both the %CRTDDS_WRITE
macro to create the define.xml file, and the %CSTUTILXMLVALIDATE macro
to validate the syntax of the generated define.xml file. For
more information about the %CSTUTILXMLVALIDATE macro, see Validation of XML-Based Standards.
The SASReferences Data Set
As a part of
each SAS Clinical Standards Toolkit process setup, a valid SASReferences
data set is required. It references the input files that are needed,
the librefs and filenames to use, and the names and locations of data
sets to be created by the process. It can be modified to point to
study-specific files. For
an explanation of the SASReferences data set, see SASReferences File.
In the SASReferences
data set, there are two input file references and three output data
set references that are key to the successful completion of the create_crtdds_define.sas
driver program. Key Components of the SASReferences Data Set for the %CRTDDS_WRITE Macro lists these
files and data sets, and they are discussed in separate sections. In the sample create_crtdds_define.sas driver program,
these values are set for &studyRootPath and &studyOutputPath:
&studyRootPath=sample study library directory/cdisc-crtdds-1.0–1.7&studyOutputPath=sample study library directory/cdisc-crtdds-1.0–1.7|
Metadata Type
|
LIBNAME or Fileref to
Use
|
Reference Type
|
Path
|
Name of File
|
|---|---|---|---|---|
|
Input
|
||||
|
control
|
control
|
libref
|
&workpath
|
sasreferences.sas7bdat
|
|
sourcedata
|
srcdata
|
libref
|
&studyRootPath/data
|
|
|
Output
|
||||
|
referencexml
|
xslt01
|
filename
|
&studyOutputPath/sourcexml
|
define-v1-updated-html.xsl
|
|
results
|
results
|
LIBNAME
|
&studyOutputPath/results
|
write_results.sas7bdat
|
|
externalxml
|
extxml
|
filename
|
&studyOutputPath/sourcexml
|
define.xml
|
Process Inputs
Use of the control library
name that points to the path in the &workpath macro variable demonstrates
a technique of documenting the derivation of the SASReferences data
set in the SAS Work library. The driver program initiates the macro
variable &workpath with this SAS code:
%let workPath=%sysfunc(pathname(work));
The sourcedata type
is the library that contains the 39 data sets that might have been
populated by the create_crtdds_from_sdtm.sas driver program. These
metadata files are the data sets that constitute the SAS representation
of the CDISC CRT-DDS 1.0 standard. In the SAS Clinical Standards Toolkit
sample study, these data sets are read from the
sample study library directory/cdisc-crtdds-1.0–1.7/data directory.
This location is represented in the driver program by the Srcdata
library name.
Process Outputs
The externalxml type
refers to the define.xml file. This file is accessed in the driver
program from the extxml filename statement, and is written to the
sample study library directory/cdisc-crtdds-1.0–1.7/sourcexml directory.
The referencexml type
can serve as either an input or output file reference. If the path
and filename are not specified, the %CRTDDS_WRITE macro interprets
the _cstCreateDisplayStyleSheet=1 parameter to indicate the default
style sheet that is provided by the SAS Clinical Standards Toolkit
in the global standards library. If a path and filename are specified,
the referencexml type serves as an output file reference for the %CRTDDS_WRITE
macro. The default style sheet is copied from the global standards
library to the path and filename that are specified.
The results type refers
to the write_results data set that documents the results of the create_crtdds_define.sas
driver program. In the SAS Clinical Standards Toolkit CDISC CRT-DDS
folder hierarchy, this information is written to the
sample study library directory/cdisc-crtdds-1.0–1.7/results directory.
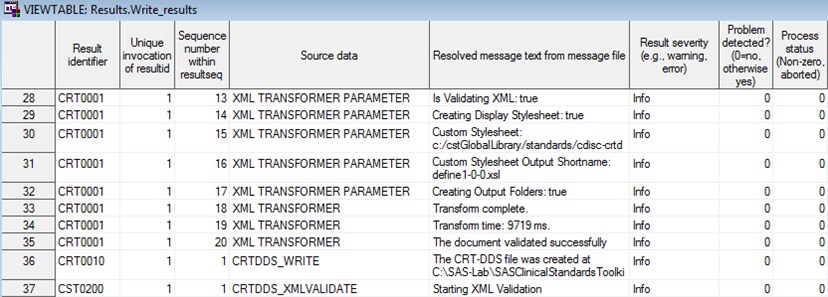
Process Results
Inclusion of the results
record (row) in the SASReferences data set indicates that the process
results are to be copied to a write_results data set located in the
specified SAS library.
Example of a Partial Results Data Set from the CRT-DDS Sample
Study
Creating a define.pdf File from the SAS Representation of the CDISC CRT-DDS 1.0 Standard
The CDER
Data Standards Common Issues Document (Version 1.1/December
2011) states:
“A critical component
of data submission is the define file. A properly functioning define.xml
file is an important part of the submission of standardized electronic
datasets and should not be considered optional. As a transition step,
CDER prefers that sponsors submit both the define.pdf and define.xml
formats. The define.pdf is primarily for printing purposes and need
not include hyperlinks. CDER will advise when it is ready to only
receive define.xml.”
The SAS Clinical Standards
Toolkit has a macro that supports the creation of a define.pdf file
from the SAS representation of a CDISC CRT-DDS 1.0 standard. This
macro is called %CRTDDS_WRITEPDF and is located here:
global standards library directory/standards/cdisc-crtdds-1.0-1.7/macrosThe %CRTDDS_WRITEPDF
macro supports the creation of a define.pdf file for the CDISC ADaM,
SDTM, and SEND standards. The contents of the sections (which attributes
are printed) is based on the Study Data Tabulation Model Metadata
Submission Guidelines (SDTM-MSG) (http://www.cdisc.org/sdtm, 2011-12-31).
The define.pdf file
has an optional table of contents and these sections:
-
Dataset level metadata
-
Variable level metadata
-
Value level metadata
-
Algorithms (Computational Methods)
-
Controlled Terminology
The following parameters
are the most important parameters for the %CRTDDS_WRITEPDF macro:
-
_cstCDISCStandardThe CDISC standard for which the define.pdf is created. Valid values: SDTM, SEND, and ADAM. The default is SDTM.
-
_cstSourceLibThe library that contains the CRT-DDS SAS data sets. If not provided, the code looks in SASReferences for type=sourcedata.
-
_cstReportOutputThe name of the PDF to create. If not provided, the code looks in SASReferences for type=report.
-
_cstLinksIndicates whether the macro creates internal hyperlinks in the PDF. Valid values: Y or N. The default is N.
-
_cstTOCIndicates that the macro creates a table of contents in the PDF. Valid values: Y or N. The default is N.
Two sample driver programs
are provided with the SAS Clinical Standards Toolkit to demonstrate
the use of the %CRTDDS_WRITEPDF macro:
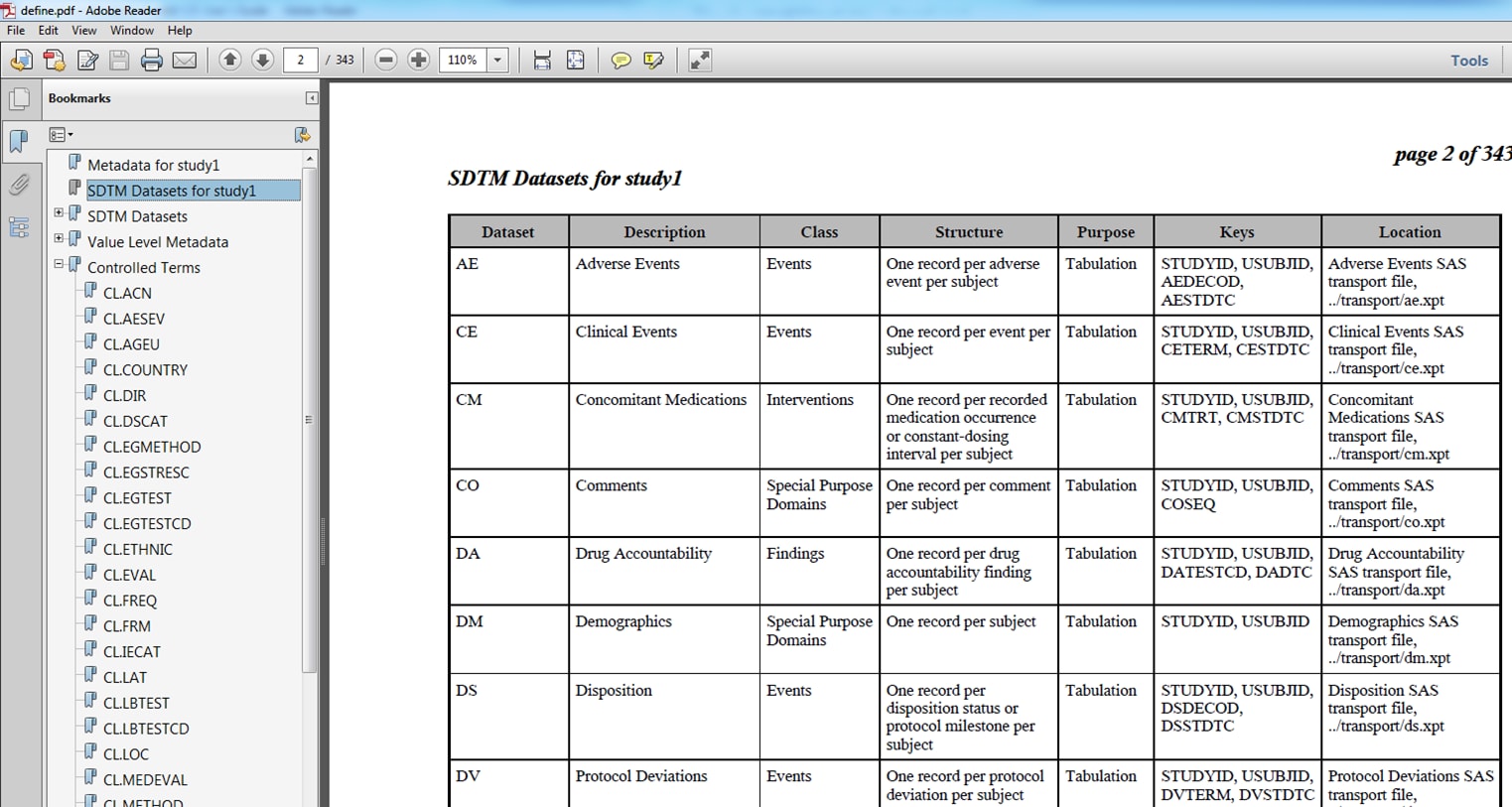
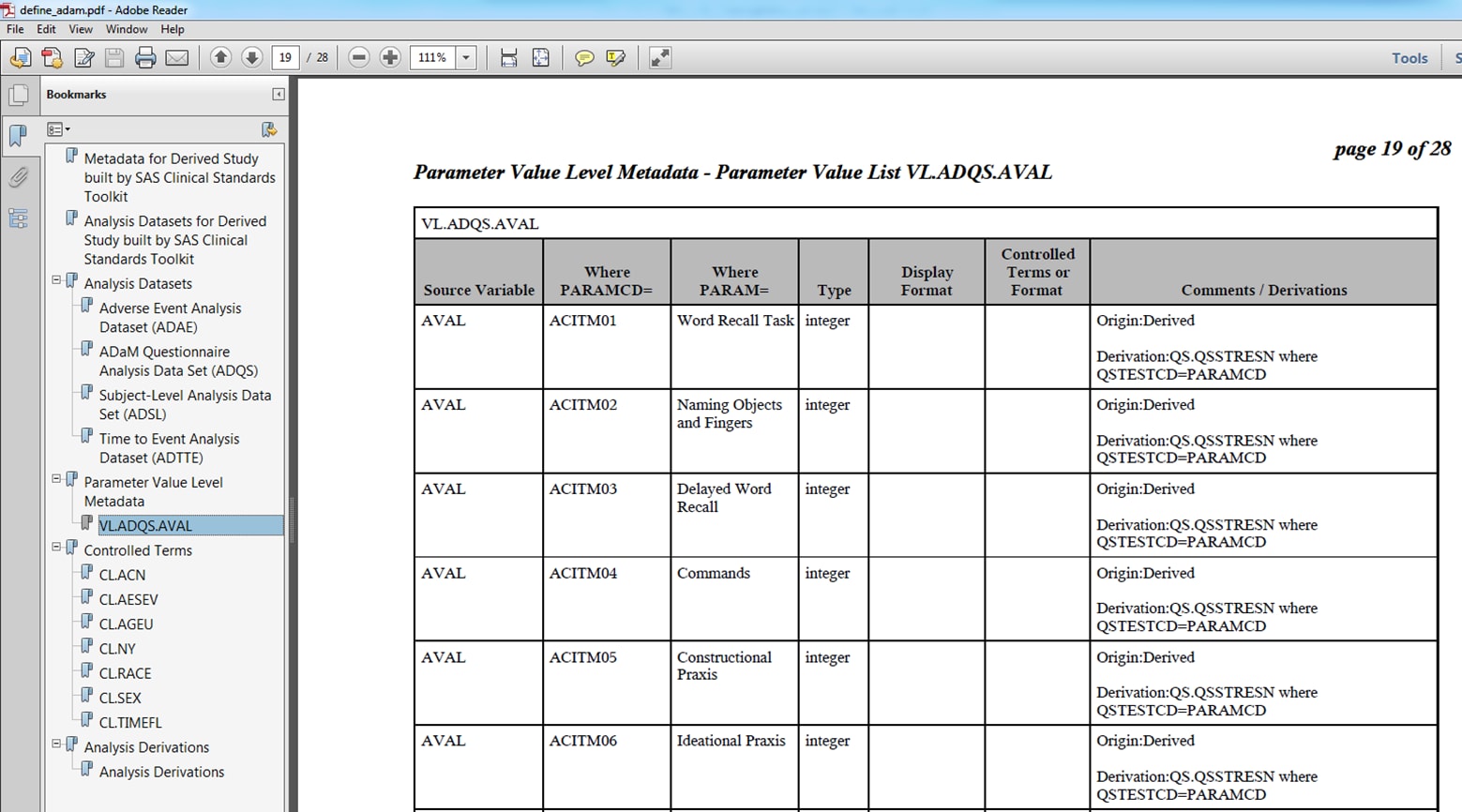
sample study library directory/cdisc-crtdds-1.0-1.7/programs/create_crtdds_define_pdf.sassample study library directory/cdisc-crtdds-1.0-1.7/programs/create_crtdds_define_pdf_adam.sasThe following displays
show examples of define.pdf files that were created by the %CRTDDS_WRITEPDF
macro:
Example define.pdf File for SDTM
Example define.pdf File for ADaM
Creating a CDISC Define-XML 2.0 define.xml File (Including Analysis Results Metadata 1.0)
There are three key
macros that are provided with the SAS Clinical Standards Toolkit that
support creation of a CDISC Define-XML 2.0 define.xml file. The three
macros are listed in the order in which they are executed:
-
The %DEFINE_SOURCETODEFINE macro creates the tables for the SAS representation of the CDISC Define-XML 2.0 files from study metadata. This macro, using SDTM or ADaM table metadata and column metadata as its source, populates a subset of the Define-XML 2.0 data sets.
-
The %DEFINE_WRITE macro creates the define.xml file from the SAS representation of the CDISC Define-XML 2.0 files.
-
The %CSTUTILXMLVALIDATE macro validates that the XML file is structurally and syntactically correct according to the XML schema for the CDISC Define-XML 2.0 standard.
These macros are called
by driver programs that are responsible for properly setting up each
SAS Clinical Standards Toolkit process to perform a specific SAS Clinical
Standards Toolkit task. Several sample driver programs are provided
with the SAS Clinical Standards Toolkit CDISC Define-XML 2.0 standard
related to the creation of the define.xml file.
Here is the purpose
of each of these driver programs:
-
The create_sasdefine_from_source.sas driver program sets up the required metadata and SASReferences data set for the sample study. It runs the %DEFINE_SOURCETODEFINE macro. It creates the SAS representation of the CDISC Define-XML 2.0 data sets from the sample study data sets.
-
The create_definexml.sas driver program creates the CDISC Define-XML 2.0 define.xml file. It runs the %DEFINE_WRITE and %CSTUTILXMLVALIDATE macros. This driver program creates and validates the XML syntax for the define.xml file.
Note: The create_definexml_from_source.sas
and create_definexml_from_source_adam.sas driver programs combine
the two purposes into one driver program.
These driver programs
are examples that are provided with the SAS Clinical Standards Toolkit.
You can use these driver programs or create your own. The names of
these driver programs are not important. However, the content is important
and demonstrates how the various SAS Clinical Standards Toolkit framework
macros are used to generate the required metadata files.
The driver programs
create a define.xml file based on SDTM or ADaM metadata.
Sample Driver Program: create_sasdefine_from_source.sas
Overview
The create_sasdefine_from_source.sas
driver program sets up the required environment variables and library
references to initiate the %DEFINE_SOURCETODEFINE macro. This macro
extracts data from the SDTM or ADaM metadata files. (For
more information about the source_tables and source_columns data sets,
see Source Metadata.) Depending on the available source information, the macro
attempts to convert the information into the tables that represent
the SAS interpretation of the CDISC Define-XML 2.0 model.
When the macro parameter
_cstFullModel has the value
N, only
the 31 Define-XML 2.0 core tables are created. Otherwise, all 46 tables
in the Define-XML 2.0 reference standard are created, but only those
tables with available data are populated. The other tables contain
zero observations. When the macro parameter _cstCheckLengths has the
value Y, the macro checks the actual value lengths of variables with
DataType=text against the lengths defined in the metadata templates.
If the lengths are short, a warning is written to the log file and
the Results data set.
Note: For more information about
the %DEFINE_SOURCETODEFINE macro, see the SAS Clinical Standards Toolkit: Macro API Documentation.
Here is an example of
a call to the %DEFINE_SOURCETODEFINE macro:
%define_sourcetodefine( _cstOutLib=srcdata, _cstSourceStudy=sampdata.source_study, _cstSourceTables=sampdata.source_tables, _cstSourceColumns=sampdata.source_columns, _cstSourceCodeLists=sampdata.source_codelists, _cstSourceDocuments=sampdata.source_documents, _cstSourceValues=sampdata.source_values, _cstFullModel=N, _cstCheckLengths=Y, _cstLang=en );
In this example, the
%DEFINE_SOURCETODEFINE macro writes all of the Define-XML 2.0 tables
to the Srcdata library.
Here is an example that
uses analysis results metadata:
%define_sourcetodefine(
_cstOutLib=srcdata,
_cstSourceStudy=sampdata.source_study,
_cstSourceTables=sampdata.source_tables,
_cstSourceColumns=sampdata.source_columns,
_cstSourceCodeLists=sampdata.source_codelists,
_cstSourceDocuments=sampdata.source_documents,
_cstSourceValues=sampdata.source_values,
_cstSourceAnalysisResults=sampdata.source_analysisresults,
_cstFullModel=N,
_cstCheckLengths=Y,
_cstLang=en
);In this example, eight
extra tables are created with metadata for analysis results.
The create_sasdefine_from_source.sas
driver program is provided with the SAS Clinical Standards Toolkit,
and it is ready to run on any of the SDTM or ADaM sample studies.
The driver program can be run interactively or in batch. To run the
driver program interactively, start a SAS session, and load the driver
program into the SAS editor.
The driver program is
located here:
sample study library directory/cdisc-definexml-2.0.0–1.7/programsThe SASReferences Data Set
As a part of
each SAS Clinical Standards Toolkit process setup, a valid SASReferences
data set is required. It references the input files that are needed,
the librefs and filenames to use, and the names and locations of data
sets to be created by the process. It can be modified to point to
study-specific files. For
an explanation of the SASReferences data set, see SASReferences File.
In the SASReferences
data set, there are seven input file references and one output data
set reference that are key to the successful completion of the create_sasdefine_from_source.sas
driver program. Key Components of the SASReferences Data Set for the create_sasdefine_from_source.sas
Driver Program lists these
files and data sets, and they are discussed in separate sections. In the sample create_sasdefine_from_source.sas driver program,
these values are set for &studyRootPath and &studyOutputPath:
&studyRootPath=sample study library directory/cdisc-definexml-2.0.0–1.7/sascstdemodata&studyOutputPath=sample study library directory/cdisc-definexml-2.0.0–1.7Here is the specification
of &_cstSrcMetaDataFolder in the SASReferences data set in the
create_sasdefine_from_source.sas driver program:
&_cstSrcMetaDataFolder=%lowcase(&_cstTrgStandard)-&_cstTrgStandardVersion/metadata
Here are the macro variable
assignments in the sample driver program to work with the sample SDTM
3.1.2 metadata:
%let _cstTrgStandard=CDISC-SDTM; %let _cstTrgStandardVersion=3.1.2;
Here is how to use the
sample driver program create_sasdefine_from_source.sas for ADaM metadata:
%let _cstTrgStandard=CDISC-ADAM; %let _cstTrgStandardVersion=2.1;
|
Metadata Type
|
SAS LIBNAME or Fileref
to Use
|
Reference Type
|
Path
|
Name of File
|
|---|---|---|---|---|
|
Input
|
||||
|
sourcemetadata
|
sampdata
|
libref
|
&studyRootPath/ &_cstSrcMetaDataFolder
|
source_study
|
|
sourcemetadata
|
sampdata
|
libref
|
&studyRootPath/ &_cstSrcMetaDataFolder
|
source_tables
|
|
sourcemetadata
|
sampdata
|
libref
|
&studyRootPath/ &_cstSrcMetaDataFolder
|
source_colums
|
|
sourcemetadata
|
sampdata
|
libref
|
&studyRootPath/ &_cstSrcMetaDataFolder
|
source_codelists
|
|
sourcemetadata
|
sampdata
|
libref
|
&studyRootPath/ &_cstSrcMetaDataFolder
|
source_values
|
|
sourcemetadata
|
sampdata
|
libref
|
&studyRootPath/ &_cstSrcMetaDataFolder
|
source_documents
|
|
sourcemetadata
|
sampdata
|
libref
|
&studyRootPath/ &_cstSrcMetaDataFolder
|
source_analysisresults
|
|
Output
|
||||
|
sourcedata
|
srcdata
|
libref
|
&studyOutputPath/data/%lowcase(&_cstTrgStandard)-&_cstTrgStandardVersion
|
|
Process Inputs
The sourcemetadata type
refers to the data sets that contain the SDTM study metadata: source_study,
source_tables, source_columns, source_values, source_codelists, source_documents,
and source_analysisresults. . These data sets are stored in the same
library.
The sample create_sasdefine_from_source.sas
driver program provided with the SAS Clinical Standards Toolkit references
a source CDISC SDTM 3.1.2 study. So, the source_tables data set contains
SDTM 3.1.2 metadata about each standard domain defined in the CDISC
SDTM Implementation Guide V3.1.2 and includes any customizations
that you have added. The source_columns data set contains similar
metadata but it is at the column level. The source_values data set
contains Value Level metadata. The source_analysisresults data set
would typically only be referenced in a CDISC ADaM study.The source
metadata is read from this location:
sample study library directory/cdisc-definexml-2.0.0–1.7/sascstdemodata/cdisc-sdtm-3.1.2/metadataThis location is represented
in the driver program by the sampdata library name.
A source study data
set (source_study) can have only one record, and it is required by
this macro. The following table lists the variables that are required
in this data set:
|
Variable*
|
Required
|
Description
|
|---|---|---|
|
SASref
|
Yes
|
The reference that ties
the study name to the corresponding domains that are associated with
this study in the source_tables and source_columns data sets.
|
|
StudyName
|
Yes
|
The name of the study.
This value is used to populate the srcdata.study.studyname column.
|
|
StudyDescription
|
Yes
|
The description of the
study. This value is used to populate the srcdata.study.studydescription
column.
Note: You cannot use commas, semicolons,
or quotation marks in the description.
|
|
ProtocolName
|
Yes
|
The name of the protocol
for the study. This value is used to populate the srcdata.study.protocolname
column.
|
|
StudyVersion
|
Yes
|
The name of the define
document to create. This value is used to populate the srcdata.metadataversion.oid
column.
|
|
FormalStandardVersion
|
Yes
|
The formal version of
the standard as used in Define-XML 2.0. This value is used to populate
the srcdata.definedocument.standardversion column. (For example, 3.1.2.)
|
|
FormalStandardName
|
Yes
|
The formal name of the
standard as used in Define-XML 2.0. This value is used to populate
the srcdata.definedocument.standardname column. (For example, SDTM-IG.)
|
|
Standard
|
Yes
|
The name of the standard
in the SAS Clinical Standards Toolkit. (For example, CDISC-SDTM.)
|
|
StandardVersion
|
Yes
|
The version of the standard
in the SAS Clinical Standards Toolkit. (For example, 3.1.2.)
|
*All variables are required
to be non-blank.
Only a single study
can be referenced in a source study data set. The %DEFINE_SOURCETODEFINE
macro selects records from only the source_tables, source_colums,
source_codelists, source_values, source_documents, and source_analysisresults
data sets whose StudyVersion column value is equal to the value of
the StudyVersion column in the source_study data set.
Process Outputs
The sourcedata type
is the library where the metadata files are created. These metadata
files are the data sets that constitute the SAS representation of
the CDISC Define-XML 2.0 standard. The create_sasdefine_from_source.sas
driver program creates 46 or 31 data sets, depending on the value
of the _cstFullModel macro parameter. Most of these data sets have
zero observations because there is no default SDTM metadata source.
In the SAS Clinical Standards Toolkit sample driver program create_sasdefine_from_source.sas,
these data sets are written to this location:
sample study library directory/cdisc-definexml–2.0.0-1.7/data/cdisc-sdtm-3.1.2This location is represented
in the driver program by the srcdata library name.
Process Results
When the driver program
finishes running, the sourcetodefine_results data set is created in
the Results library. This data set contains informational, warning,
and error messages that were generated by the driver program.
Example of a Partial Results Data Set from Define-XML 2.0 Sample
Study
Sample Driver Program: create_definexml.sas
Overview
The create_definexml.sas
driver program sets up the required environment variables and library
references to initiate the %DEFINE_WRITE macro. This macro reads the
data sets that comprise the SAS representation of the CDISC Define-XML
2.0 model, and it converts that information to the required XML structure.
If source metadata or data are missing, then empty elements and attributes
are not created in the XML file. The inputs and outputs are specified
in the SASReferences data set.
Note: For more information about
the %DEFINE_WRITE macro, see the SAS Clinical Standards Toolkit: Macro API Documentation.
Here is an example of
a call to the %DEFINE_WRITE macro:
%define_write(_cstCreateDisplayStyleSheet=1,
_cstOutputEncoding=UTF-8,
_cstResultsOverrideDS=&_cstResultsDS);In this example, a default
style sheet is generated in the same directory as the XML output based
on the information in the SASReferences data set. XML encoding is
set to UTF-16, and process results are written to the default &_cstResultsDS
data set.
Here is the call to
the macro from the sample create_definexml.sas driver program:
%define_write(_cstCreateDisplayStyleSheet=1);
The call creates a display
style sheet and uses default values for the parameters.
The create_definexml.sas
driver program is ready to run on any of the CDISC SDTM sample studies.
The driver program can be run interactively or in batch.
The driver program is
located here:
sample study library directory/cdisc-definexml-2.0.0–1.7/programs Multiple tasks can be
executed in any SAS Clinical Standards Toolkit driver program. The
create_definexml.sas driver program calls both the %DEFINE_WRITE macro
to create the Define-XML file and the %CSTUTILXMLVALIDATE macro to
validate the syntax of the generated Define-XML file. For
more information about the %CSTUTILXMLVALIDATE macro, see Validating an XML File against an XML Schema: %CSTUTILXMLVALIDATE Macro.
The SASReferences Data Set
As a part of
each SAS Clinical Standards Toolkit process setup, a valid SASReferences
data set is required. It references the input files that are needed,
the librefs and filenames to use, and the names and locations of data
sets to be created by the process. It can be modified to point to
study-specific files. For
an explanation of the SASReferences data set, see SASReferences File.
In the SASReferences
data set, there are two input file references and three output data
set references that are key to the successful completion of the create_definexml.sas
driver program. Key Components of the SASReferences Data Set for the %DEFINE_WRITE Macro lists these
files and data sets, and they are discussed in separate sections. In the sample create_definexml.sas driver program, these
values are set for &studyRootPath and &studyOutputPath:
&studyRootPath=sample study library directory/cdisc-definexml-2.0.0–1.7&studyOutputPath=sample study library directory/cdisc-definexml-2.0.0–1.7|
Metadata Type
|
LIBNAME or Fileref to
Use
|
Reference Type
|
Path
|
Name of File
|
|---|---|---|---|---|
|
Input
|
||||
|
control
|
control
|
libref
|
&workpath
|
sasreferences
|
|
sourcedata
|
srcdata
|
libref
|
&studyRootPath/data/&_cstSrcDataFolder
|
|
|
Output
|
||||
|
referencexml
|
xslt01
|
filename
|
define-2-0-0.xsl
|
|
|
results
|
results
|
libref
|
&studyOutputPath/results
|
write_results
|
|
externalxml
|
extxml
|
filename
|
&studyOutputPath/sourcexml
|
&_cstDefineFile..xml
|
|
report
|
html
|
filename
|
&studyOutputPath/sourcexml
|
&_cstDefineFile..html
|
Here is the specification
of &_cstSrcMetaDataFolder in the SASReferences data set in the
create_sasdefine_from_source.sas driver program:
_cstSrcDataFolder=%lowcase(&_cstTrgStandard)-&_cstTrgStandardVersion
Here are the variable
assignments in the sample driver program to work with the sample SDTM
3.1.2 metadata:
%let _cstTrgStandard=CDISC-SDTM; %let _cstTrgStandardVersion=3.1.2; %let _cstDefineFile=define-sdtm-3.1.2.xml;
Process Inputs
Use of the control library
name that points to the path in the &workpath macro variable demonstrates
a technique of documenting the derivation of the SASReferences data
set in the SAS Work library. The driver program initiates the macro
variable &workpath with this SAS code:
%let workPath=%sysfunc(pathname(work));
The sourcedata type
is the library that contains the Define-XML data sets that might have
been populated by the create_sasdefine_from_source.sas driver program.
These metadata files are the data sets that constitute the SAS representation
of the CDISC Define-XML 2.0 standard. In the SAS Clinical Standards
Toolkit sample study, these data sets are read from the
sample study library directory/cdisc-definexml–2.0.0-1.7/data/cdisc-sdtm-3.1.2 directory.
This location is represented in the driver program by the Srcdata
library name.
Process Outputs
The externalxml type
refers to the define-sdtm-3.1.2.xml file. This file is accessed in
the driver program from the extxml filename statement, and is written
to the
sample study library directory/cdisc-definexml–2.0–1.7/sourcexml directory.
The referencexml type
can serve as either an input or output file reference. If the path
and filename are not specified, the %DEFINE_WRITE macro interprets
the _cstCreateDisplayStyleSheet=1 parameter to indicate the default
style sheet that is provided by the SAS Clinical Standards Toolkit
in the global standards library. If a path and filename are specified,
the referencexml type serves as an output file reference for the %DEFINE_WRITE
macro. The default style sheet is copied from the global standards
library to the path and filename that are specified.
The results type refers
to the write_results data set that documents the results of the create_definexml.sas
driver program. In the SAS Clinical Standards Toolkit CDISC Define-XML
folder hierarchy, this information is written to the
sample study library directory/cdisc-definexml–2.0-1.7/results directory.
In Microsoft Windows,
the define-sdtm-3.1.2.xml file can be viewed by double-clicking it
in the SAS Program Editor. This renders the file in your default web
browser or in any other application that has been associated with
XML files.
On UNIX, if you have
not set up your browser configuration in SAS, you need to copy define-sdtm-3.1.2.xml
and define2-0-0.xsl to an environment where you can display the XML
file in a web browser.
Note: The style sheet information
in define2-0-0.xsl is not guaranteed to work for all browser types
and versions to produce the correct HTML. But, it does work with Internet
Explorer 6.0 and higher. The Chrome browser, for example, does not
allow local XML and XSLT processing.
The sample driver program
also creates the HTML rendition in the same folder as the XML file
using this code:
proc xsl in=extxml xsl=xslt01 out=html; run;
Instead of opening the
XML file in a browser and letting the browser use the XSL file to
render the HTML, you can directly open the HTML file.
Depending on your browser,
you might see a security warning because the style sheet uses JavaScript.
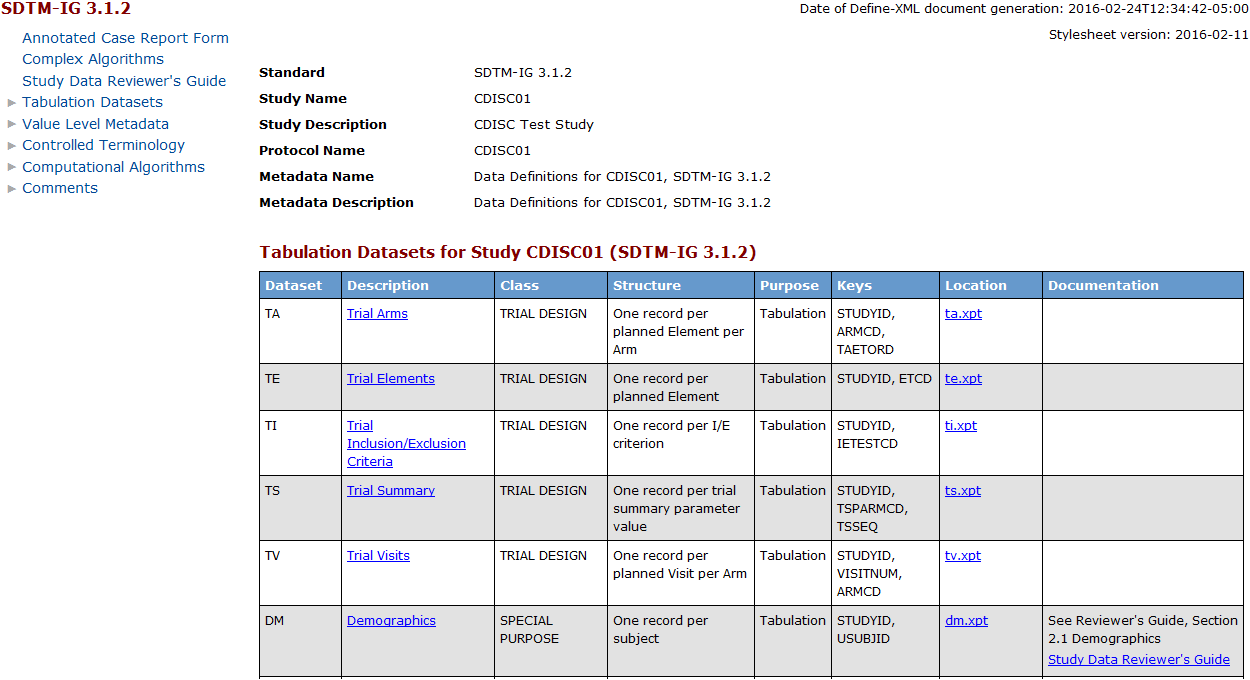
The following display
shows the define-sdtm-3.1.2.xml file in a web browser:
define-sdtm-3.1.2.xml File in a Web Browser
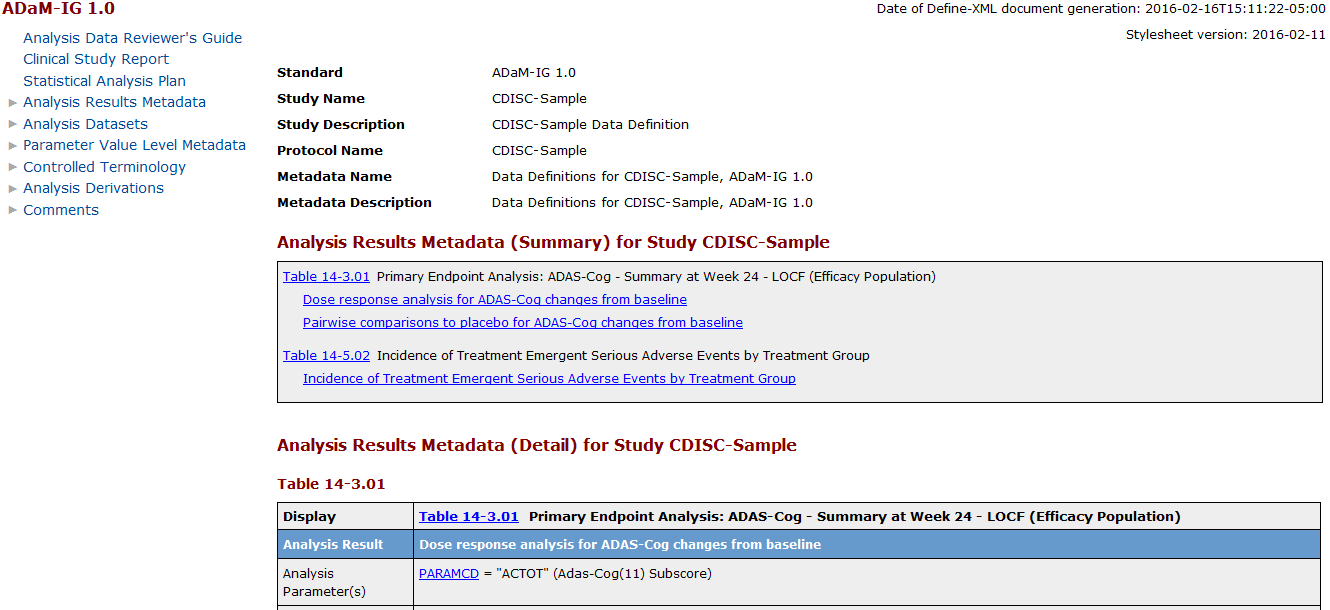
The following display
shows the define-adam-2.1.xml file in a web browser:
define-adam-2.1.xml File in a Web Browser
Process Results
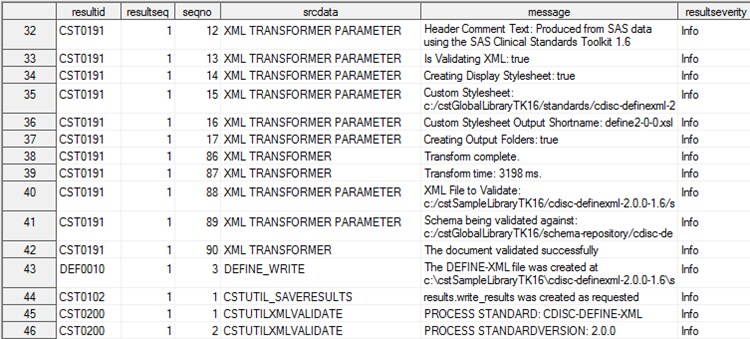
Inclusion of the results
record (row) in the SASReferences data set indicates that the process
results are to be copied to a write_results data set located in the
specified SAS library.
Example of a Partial Results Data Set from the Define-XML 2.0
Sample Study
Creating a CDISC ODM XML File
Note: The process to create a CDISC
ODM XML file is the same for all ODM versions that are supported by
the SAS Clinical Standards Toolkit. The process is explained using
ODM version 1.3.0.
There are several key
macros that are provided with the SAS Clinical Standards Toolkit that
support the creation of an ODM XML file. The macros are listed in
the order in which they are executed:
-
The %ODM_VALIDATE macro submits a set of validation checks based on what is defined in the Validation Control data set to validate the referenced SAS representation of each ODM XML file.
-
The %ODM_WRITE macro creates the ODM XML file from the SAS representation of the ODM files and validates that the XML file is structurally and syntactically correct. This macro is important if you customize the XML file outside of the workflow.
-
The %CSTUTILXMLVALIDATE macro validates that the XML file is structurally and syntactically correct, according to the XML schema for the ODM standard. This macro is important if you customize the ODM XML file outside of the workflow.
These macros are called
by driver programs that are responsible for properly setting up each
SAS Clinical Standards Toolkit process to perform a specific SAS Clinical
Standards Toolkit task. Two sample driver programs are provided with
the SAS Clinical Standards Toolkit CDISC ODM standard related to the
creation of the XML file.
Here is the purpose
of each of these driver programs:
-
The validate_odm_data.sas driver program validates the SAS representation of the ODM data sets based on the selected ODM validation checks. This driver program can be run multiple times until data validation has been reconciled.
-
The create_odmxml.sas driver program calls the %ODM_WRITE macro to create the XML file. This driver program creates and validates the syntax for the XML file.
These driver programs
are examples that are provided with the SAS Clinical Standards Toolkit.
You can use these driver programs or create your own. The names of
these driver programs are not important. However, the content is important
and demonstrates how the various SAS Clinical Standards Toolkit framework
macros are used to generate the required metadata files.
Sample Driver Program: create_odmxml.sas
Overview
The create_odmxml.sas
driver program sets up the required environment variables and library
references to initiate the %ODM_WRITE macro. This macro reads the
66 data sets that comprise the default SAS representation of the CDISC
ODM 1.3.0 model, and it converts that information to the required
ODM XML structure. If source metadata or data are missing, then empty
elements and attributes are not created in the ODM XML file. The inputs
and outputs are specified in the SASRferences data set.
For more information
about the %ODM_WRITE macro, see the SAS Clinical Standards Toolkit: Macro API Documentation.
Here is an example of
a call to the %ODM_WRITE macro:
%odm_write(_cstOutputEncoding=UTF-16, _cstResultsOverrideDS=&_cstResultsDS);
In this example, no
default style sheet is generated for the XML output, XML encoding
is set to UTF-16, and process results are written to the default &_cstResultsDS
data set.
Here is the call to
the macro from the sample create_odmxml.sas driver program:
%odm_write();
The call uses default
values for the parameters. The create_odmxml.sas driver program is
ready to run on the CDISC ODM sample study provided with the SAS Clinical
Standards Toolkit. The driver program can be run interactively or
in batch.
The driver program is
located here:
sample study library directory/cdisc-odm-1.3.0–1.7/programsThe SASReferences Data Set
As a part of
each SAS Clinical Standards Toolkit process setup, a valid SASReferences
data set is required. It references the input files that are needed,
the librefs and filenames to use, and the names and locations of data
sets to be created by the process. It can be modified to point to
study-specific files. For
an explanation of the SASReferences data set, see SASReferences File.
In the SASReferences
data set, there are one input file reference and two output data set
references that are key to the successful completion of the create_odmxml.sas
driver program. Key Components of the SASReferences Data Set for the %ODM_WRITE Macro lists these
files and data sets, and they are discussed in separate sections. In the sample create_odmxml.sas driver program, these values
are set for &studyRootPath and &studyOutputPath:
&studyRootPath=sample study library directory/cdisc-odm-1.3.0–1.7&studyOutputPath=sample study library directory/cdisc-odm-1.3.0–1.7|
Metadata Type
|
SAS LIBNAME or Fileref
to Use
|
Reference Type
|
Path
|
Name of File
|
|---|---|---|---|---|
|
Input
|
||||
|
sourcedata
|
srcdata
|
libref
|
&studyRootPath/data
|
|
|
Output
|
||||
|
results
|
results
|
libref
|
&studyOutputPath/results
|
write_results.sas7bdat
|
|
externalxml
|
extxml
|
filename
|
&studyOutputPath/sourcexml
|
odm_sample_out.xml
|
Process Inputs
The sourcedata type
is the library that contains the default 66 data sets that comprise
the SAS representation of an ODM XML file. These data sets might have
been populated by a previous odm_read task, or you might have processes
in place that build these data sets from source files. In the SAS
Clinical Standards Toolkit sample study, these data sets are read
from the
sample study library directory/cdisc-odm-1.3.0–1.7/data directory.
This location is represented in the driver program by the Srcdata
library name.
Process Outputs
The externalxml type
refers to the ODM XML file that is to be derived by the process. This
file is accessed in the driver program from the extxml filename statement,
and is written to the
sample study library directory/cdisc-odm-1.3.0–1.7/sourcexml directory.
Note: Unlike CDISC CRT-DDS or CDISC
Define-XML, CDISC does not supply a default style sheet for ODM and
one is not provided as part of the SAS Clinical Standards Toolkit.
However, you can use the %ODM_WRITE macro, which provides the _cstCreateDisplayStyleSheet
parameter, to use information that you provide in the Metadata Type
referencexml record of the SASReferences file.
The results type refers
to the write_results data set that documents the results of the create_odmxml
driver program. In the SAS Clinical Standards Toolkit CDISC CRT-DDS
folder hierarchy, this information is written to this location:
sample study library directory/cdisc-odm-1.3.0–1.7/resultsProcess Results
Inclusion of the results
record (row) in the SASReferences data set indicates that the process
results are to be copied to a write_results data set located in the
specified SAS library.
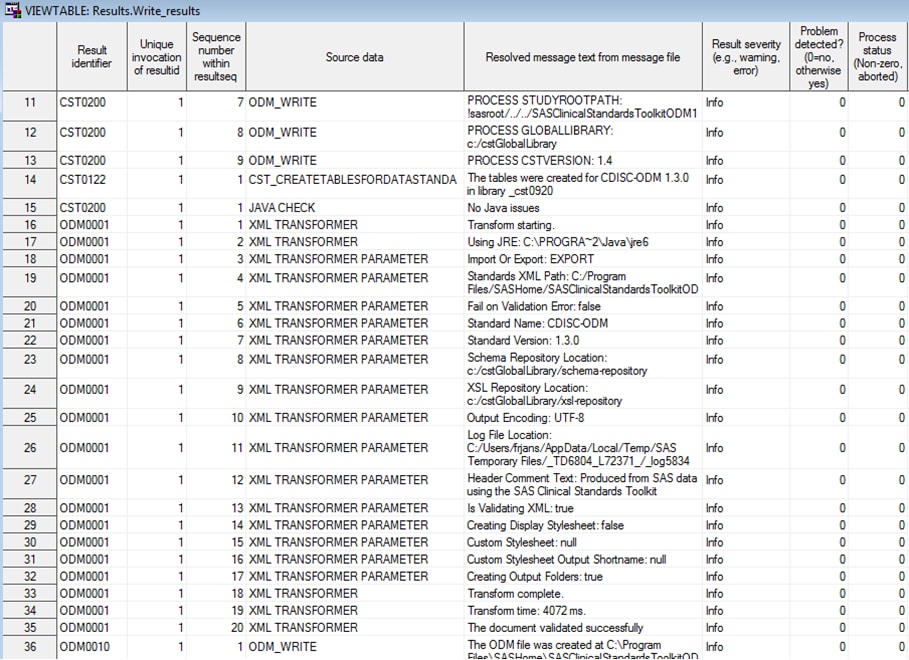
Example of a Partial Results Data Set from the ODM Sample Data
Hierarchy
Copyright © SAS Institute Inc. All Rights Reserved.