Validating the CDISC ADaM Data Sets
Assumption
You have a library of
CDISC ADaM SAS data sets (for the purposes of this example, it is
derived from a library of CDISC SDTM 3.1.2 domains). Derivation of
ADaM analysis files from SDTM domains is not a supported function
in the SAS Clinical Standards Toolkit. Products such as SAS Clinical
Data Integration can be used to create these mapping processes and
transformation processes.
Step 1: Derive Metadata about Your Source Data
Before you can validate
your ADaM data sets, you must derive a set of metadata that describes
your library of analysis data sets. In the SAS Clinical Standards
Toolkit, the metadata that describes your study data sets and columns
is generally referred to as source metadata.
To help derive this metadata, the SAS Clinical Standards Toolkit provides
a sample driver program (create_sourcemetadata.sas) that calls the
SAS macro adamutil_createsrcmetafromsaslib.sas found in the
global standards library directory/standards/cdisc-adam-2.1-1.6/macros directory.
This macro uses Base SAS metadata (PROC CONTENTS output) and reference
metadata (provided by SAS) describing the CDISC ADaM standard to initialize
the source metadata. You might need to augment or modify this approach
based on other metadata that you have available or other processes
that you adopt.
Step 2: Build Your Own Driver Program
The sample driver that
can be run to demonstrate the ADaM validation process is validate_data.sas.
Use this driver as a sample to build your own driver program, specifying
the locations of source data and metadata. Take note of the SASReferences
data set created in the validate_data driver. It references both SDTM
and ADaM data and metadata, as well as ADaM controlled terminology.
Reference to the SDTM metadata supports comparison of ADaM column
metadata with SDTM column metadata for those columns derived directly
from SDTM. (See Assumption.)
Step 3: Submit the Modified Driver Program
The SAS Clinical Standards
Toolkit validation processes generally create two types of output
data sets: validation results and validation metrics. The names and
locations of these SAS data sets depend on your SASReferences specifications
for results management.
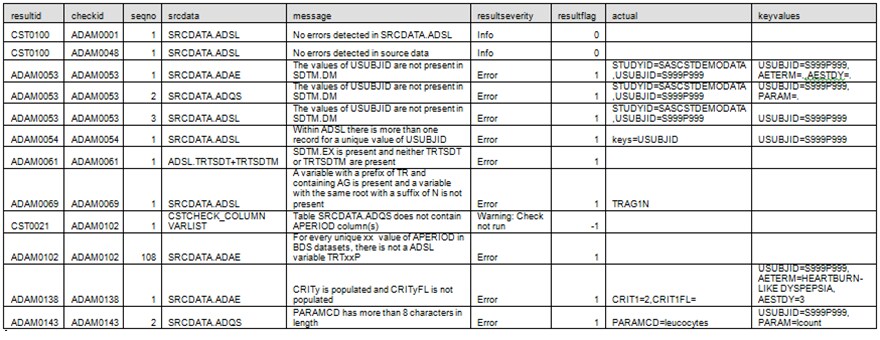
The validation results
are representative of the range of validation results one might see,
from no reported errors (such as ADAM0001) to multiple errors detected
(such as ADAM0053) to an inability to run a specific check because
of a lack of data or metadata (such as ADAM0102). The validation metrics
output data set attempts to summarize the validation results and provide
a denominator for each check.
Copyright © SAS Institute Inc. All rights reserved.