Assessing CDISC SDTM Compliance
Overview
Assess CDISC SDTM Compliance
To assess CDISC SDTM
compliance, perform the following steps:
-
-
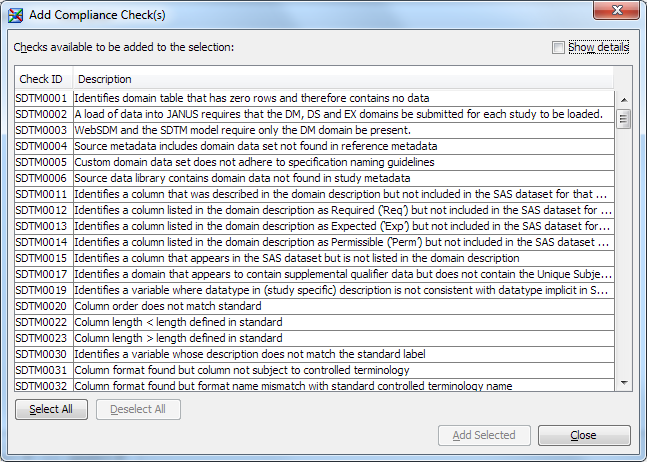
Note: If you imported the CDISC SDTM 3.1.2 data standard, then compliance checks for this data standard also appear in the Standard column in the Checks tab. A check that has the value
CDISC-SDTMapplies to both the CDISC SDTM 3.1.1 and CDISC SDTM 3.1.2 standards. A check that has the valueCDISC-SDTM 3.1.2applies to the CDISC SDTM 3.1.2 standard only. -
Note: Some check IDs are listed more than once. When you select Show details, you can view information that makes the individual compliance check unique. For example, consider the check ID SDTM0001: To be efficient, choose only a subset of the compliance checks. It is inefficient to run the same compliance checks multiple times if the only differences between the checks are severity and source.
-
You might want to connect the Results and Metrics work tables as input to the code that will be used to generate formatted reports of the results.Note: You might encounter errors or warnings in the SAS log during job execution. These errors or warnings do not mean that validation was unsuccessful. Most errors that halt a validation are reported in the Results work table. As a general rule, the Results work table reports failures and provides information about the cause of the failures. For more information about validation, see the SAS Clinical Standards Toolkit: User Guide.
-
For information, see Reporting on the Results of a Transformation.