Sample 59221: Bayesian Hierarchical Modeling for Meta-Analysis
 |  |  |  |
Overview
Meta-analysis is an important technique that combines information from different studies. When you have no prior information for thinking any particular study is different from another, you can treat Bayesian meta-analysis as a hierarchical model. This example illustrates two different applications of hierarchical modeling to a meta-analysis in medicine. The first application uses a normal approximation to the likelihood, and the second application uses the exact binomial likelihood.
Analysis
Consider the data from Yusuf et al. (1985), which summarize mortality after myocardial infarction from 22 studies. For each study, the data are in the form of  tables that consist of patients who are randomly assigned to receive beta-blockers or placebo. For study
tables that consist of patients who are randomly assigned to receive beta-blockers or placebo. For study  , suppose that trt
, suppose that trt is the number of deaths out of trtN
is the number of deaths out of trtN patients in the treatment group and ctrl
patients in the treatment group and ctrl is the number of deaths out of ctrlN
is the number of deaths out of ctrlN patients in the control group.
patients in the control group.
The following statements read the data into SAS and create the Multistudies data set:
data multistudies; input studyid ctrl ctrlN trt trtN; datalines; 1 3 39 3 38 2 14 116 7 114 3 11 93 5 69 4 127 1520 102 1533 5 27 365 28 355 6 6 52 4 59 7 152 939 98 945 8 48 471 60 632 9 37 282 25 278 10 188 1921 138 1916 11 52 583 64 873 12 47 266 45 263 13 16 293 9 291 14 45 883 57 858 15 31 147 25 154 16 38 213 33 207 17 12 122 28 251 18 6 154 8 151 19 3 134 6 174 20 40 218 32 209 21 43 364 27 391 22 39 674 22 680 ;
Suppose you want use a Bayesian random-effects model to estimate both the study-specific treatment effect and the pooled treatment effect. A Bayesian random-effects model assumes that there is no prior information for thinking that one study is different from the other. This assumption is also known as exchangeability.
Hierarchical Model Using Normal Approximation to the Likelihood
Normal approximation to binomial likelihood is a classical method that is commonly used in meta-analysis. Let  be the odds ratio for the
be the odds ratio for the  th study:
th study:
 |
You can estimate the treatment effect  ,
,  , through the means of an approximate normal distribution,
, through the means of an approximate normal distribution,
 |
where  is the study-specific effect and
is the study-specific effect and  is the known variance of
is the known variance of  . For
. For  , because all studies have large sample sizes (more than 50 persons in each group in nearly all of the studies), you can use the approximate sampling variance of
, because all studies have large sample sizes (more than 50 persons in each group in nearly all of the studies), you can use the approximate sampling variance of  :
:
 |
If the odds ratios are exchangeable between the studies, then the treatment effect in each trial can be considered to be a random quantity drawn from some population distribution. In a Bayesian framework, this means that you can place a common prior distribution on the exchangeable random-effects parameters 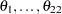 ,
,
 |
where  is the population average of the treatment effect across all studies and
is the population average of the treatment effect across all studies and  is the between-study variation. Suppose the following noninformative priors are placed on the hyperparameters
is the between-study variation. Suppose the following noninformative priors are placed on the hyperparameters  and
and  :
:
 |
 |
 |
|||
 |
 |
 |
The following DATA step creates the response variable,  , and its approximate sampling variance,
, and its approximate sampling variance,  for
for  .
.
data multistudies; set multistudies; logy=log(trt/(trtN-trt)/(ctrl/(ctrlN-ctrl))); sigma2=1/trt+1/(trtN-trt)+1/ctrl+1/(ctrlN-ctrl); run;
The following MCMC procedure statements fit the described random-effects model:
proc mcmc data=multistudies outpost=nlout seed=276 nmc=50000 thin=5 monitor=(OR Pooled); array OR[22]; parms mu tau2; prior mu ~ normal(0, sd=3); prior tau2~ igamma(0.01,s=0.01); random theta ~n(mu, var=tau2) subject=studyid; OR[studyid]=exp(theta); Pooled=exp(mu); model logy ~ n(theta, var=sigma2); run;
The PROC MCMC statement specifies the input data set as Multistudies and the output data set as Nlout, sets a seed for the random number generator, and requests a large simulation size with thinning. The MONITOR= option in the PROC MCMC statement outputs the analyses or saves the posterior samples for the specified variables. The ARRAY statement allocates an array variable OR of size 22, which is the number of random-effects parameters. The PARMS statement declares  and
and  as model parameters, and the PRIOR statements specify their prior distributions.
as model parameters, and the PRIOR statements specify their prior distributions.
The RANDOM statement declares  as random-effects parameters and specifies the normal prior distribution with hyperparameters
as random-effects parameters and specifies the normal prior distribution with hyperparameters  and
and  . The SUBJECT= option in the RANDOM statement specifies the group index as the data set variable studyid. The RANDOM statement generates 22 random-effects parameters, one for each unique value in the studyid variable. To monitor the function of 22 random-effects parameters, you must allocate an array and store the transformation of every random-effects parameter. The next programming statement, OR[studyid]=exp(theta) , does precisely this. As the procedure steps through the data set, it calculates the exponential of each
. The SUBJECT= option in the RANDOM statement specifies the group index as the data set variable studyid. The RANDOM statement generates 22 random-effects parameters, one for each unique value in the studyid variable. To monitor the function of 22 random-effects parameters, you must allocate an array and store the transformation of every random-effects parameter. The next programming statement, OR[studyid]=exp(theta) , does precisely this. As the procedure steps through the data set, it calculates the exponential of each  parameter and stores the result in the
parameter and stores the result in the  th element of the OR array according to the studyid variable value (indexing). For this statement to work, the subject index variable studyid must have an integer value (1, 2, 3, and so on). The symbol Pooled calculates the exponential of the hyperparameter
th element of the OR array according to the studyid variable value (indexing). For this statement to work, the subject index variable studyid must have an integer value (1, 2, 3, and so on). The symbol Pooled calculates the exponential of the hyperparameter  , which is the pooled treatment effect. The MODEL statement specifies the normal likelihood for the log odds ratios.
, which is the pooled treatment effect. The MODEL statement specifies the normal likelihood for the log odds ratios.
Figure 1 reports posterior summary statististics for each of the 22 odds ratios and for the Pooled estimate. The odds ratios provide the multiplicative change in the odds of mortality in the treatment group with respect to the control group. Some variation exists among the treatment effects. The pooled estimate of the odds ratio is 0.7849, indicating that mortality is less likely in the treatment groups.
| Posterior Summaries | ||||||
|---|---|---|---|---|---|---|
| Parameter | N | Mean | Standard Deviation |
Percentiles | ||
| 25% | 50% | 75% | ||||
| OR1 | 10000 | 0.8038 | 0.1471 | 0.7112 | 0.7879 | 0.8749 |
| OR2 | 10000 | 0.7545 | 0.1253 | 0.6764 | 0.7523 | 0.8285 |
| OR3 | 10000 | 0.7752 | 0.1313 | 0.6913 | 0.7663 | 0.8494 |
| OR4 | 10000 | 0.7863 | 0.0852 | 0.7284 | 0.7809 | 0.8387 |
| OR5 | 10000 | 0.8563 | 0.1366 | 0.7629 | 0.8370 | 0.9248 |
| OR6 | 10000 | 0.7791 | 0.1370 | 0.6924 | 0.7697 | 0.8529 |
| OR7 | 10000 | 0.6829 | 0.0776 | 0.6312 | 0.6835 | 0.7349 |
| OR8 | 10000 | 0.8381 | 0.1116 | 0.7628 | 0.8269 | 0.9007 |
| OR9 | 10000 | 0.7544 | 0.1095 | 0.6829 | 0.7499 | 0.8216 |
| OR10 | 10000 | 0.7449 | 0.0705 | 0.6964 | 0.7423 | 0.7896 |
| OR11 | 10000 | 0.7978 | 0.1007 | 0.7294 | 0.7910 | 0.8572 |
| OR12 | 10000 | 0.8430 | 0.1203 | 0.7609 | 0.8293 | 0.9073 |
| OR13 | 10000 | 0.7580 | 0.1211 | 0.6797 | 0.7536 | 0.8286 |
| OR14 | 10000 | 0.9573 | 0.1570 | 0.8469 | 0.9297 | 1.0455 |
| OR15 | 10000 | 0.7793 | 0.1166 | 0.7023 | 0.7727 | 0.8458 |
| OR16 | 10000 | 0.8151 | 0.1202 | 0.7334 | 0.8052 | 0.8803 |
| OR17 | 10000 | 0.8454 | 0.1429 | 0.7516 | 0.8250 | 0.9157 |
| OR18 | 10000 | 0.8322 | 0.1525 | 0.7349 | 0.8122 | 0.9061 |
| OR19 | 10000 | 0.8232 | 0.1511 | 0.7284 | 0.8036 | 0.8941 |
| OR20 | 10000 | 0.7964 | 0.1168 | 0.7195 | 0.7847 | 0.8621 |
| OR21 | 10000 | 0.7202 | 0.1067 | 0.6484 | 0.7191 | 0.7879 |
| OR22 | 10000 | 0.7218 | 0.1055 | 0.6541 | 0.7222 | 0.7866 |
| Pooled | 10000 | 0.7849 | 0.0525 | 0.7498 | 0.7826 | 0.8166 |
You can use  CATER autocall macro to create a caterpillar plot of the odds ratios. The following statement takes the output from the Nlout data set and generates a caterpillar plot of all study-specific odds ratios and the pooled odds ratio:
CATER autocall macro to create a caterpillar plot of the odds ratios. The following statement takes the output from the Nlout data set and generates a caterpillar plot of all study-specific odds ratios and the pooled odds ratio:
%CATER(data=nlout, var=OR: Pooled);
Figure 2 shows the posterior means and the 95 equal-tail credible intervals of odds ratios from the 22 studies and for the pooled odds ratio. It looks like the treatment is most effective for Study 7 and least effective for Study 14. This result illustrates another advantage of the random-effects models: a fixed-effects model focuses on the coefficient effects after averaging study differences, whereas a random-effects model can help identify studies that are very different from others, such as ones with very high or low treatment effects.
equal-tail credible intervals of odds ratios from the 22 studies and for the pooled odds ratio. It looks like the treatment is most effective for Study 7 and least effective for Study 14. This result illustrates another advantage of the random-effects models: a fixed-effects model focuses on the coefficient effects after averaging study differences, whereas a random-effects model can help identify studies that are very different from others, such as ones with very high or low treatment effects.

Hierarchical Model Using Binomial Likelihood
The standard normal approximation method might not be appropriate because the approximation becomes less precise in the extreme probabilities (for example, 0,1). In the Multistudies data set, the mortality rates in the thirteenth study and nineteenth study are closest to 0 ( ). Therefore, an alternative approach is to use the exact likelihood approach as opposed the normal approximation.
). Therefore, an alternative approach is to use the exact likelihood approach as opposed the normal approximation.
Emulating the binomial model as outlined in Spiegelhalter, Abrams, and Myles (2004), you can use the following model, where the treatment and control groups have their own binomial likelihood functions, with death probabilities  and
and  in the treatment group and control group, respectively:
in the treatment group and control group, respectively:
 |
 |
 |
|||
 |
 |
 |
Let the log odds for the control group be  and let the log odds for the treatment group be
and let the log odds for the treatment group be  as follows:
as follows:
 |
Then  is the log odds ratio:
is the log odds ratio:
 |
If treatment group risks are exchangeable, then you can assume that  are random-effects parameters that are drawn from some common prior distribution:
are random-effects parameters that are drawn from some common prior distribution:
 |
If the control group risks are also exchangeable, then you can consider  as other random-effects parameters with the following common prior:
as other random-effects parameters with the following common prior:
 |
Suppose the following priors are placed on the model parameters:
 |
 |
 |
|||
 |
 |
 |
The following PROC MCMC statements fit the described model:
proc mcmc data=multistudies outpost=blout seed=126 nmc=50000 thin=5 monitor=(OR Pooled); array OR[22]; parms mu_theta mu_phi s_theta s_phi; prior mu:~ normal(0,sd=3); prior s:~ igamma(0.01,s=0.01); random theta ~n(mu_theta, var=s_theta) subject=studyid; random phi ~n(mu_phi, var=s_phi) subject=studyid; p = logistic(theta + phi); q = logistic(phi); model trt ~ binomial(trtN, p); model ctrl ~ binomial(ctrlN, q); OR[studyid]=exp(theta); Pooled=exp(mu_theta); run;
The PROC MCMC statement specifies the input data set as Multistudies and the output data set as Blout, sets a seed for the random number generator, and requests a large simulation size with thinning. The MONITOR= option in the PROC MCMC statement saves the posterior samples for the specified variables. The ARRAY statement allocates an array variable OR of size 22. The PARMS statement declares  , and
, and  as model parameters, and the PRIOR statements specify their prior distributions. The RANDOM statements declare
as model parameters, and the PRIOR statements specify their prior distributions. The RANDOM statements declare  and
and  as random-effects parameters and specify a normal prior distribution for them. The SUBJECT= option in both RANDOM statements indicates the group index as data set variable studyid. The LOGISTIC functions perform logistic transformation from the log odds of the treatment and control groups to the corresponding binomial mortality probabilities. The MODEL statements specify the binomial likelihood for the treatment and the control groups. The programming statement in the next line, OR[studyid]=exp(theta) , calculates the exponential of each ‘theta’ effect and stores the result in the OR array according to the studyid variable value (indexing). The symbol Pooled calculates the exponential of the hyperparameter
as random-effects parameters and specify a normal prior distribution for them. The SUBJECT= option in both RANDOM statements indicates the group index as data set variable studyid. The LOGISTIC functions perform logistic transformation from the log odds of the treatment and control groups to the corresponding binomial mortality probabilities. The MODEL statements specify the binomial likelihood for the treatment and the control groups. The programming statement in the next line, OR[studyid]=exp(theta) , calculates the exponential of each ‘theta’ effect and stores the result in the OR array according to the studyid variable value (indexing). The symbol Pooled calculates the exponential of the hyperparameter  , which is the pooled treatment effect.
, which is the pooled treatment effect.
Figure 3 reports posterior summary statististics for each of the 22 odds ratios.
| Posterior Summaries | ||||||
|---|---|---|---|---|---|---|
| Parameter | N | Mean | Standard Deviation |
Percentiles | ||
| 25% | 50% | 75% | ||||
| OR1 | 10000 | 0.7960 | 0.1410 | 0.7066 | 0.7867 | 0.8668 |
| OR2 | 10000 | 0.7477 | 0.1221 | 0.6698 | 0.7428 | 0.8202 |
| OR3 | 10000 | 0.7734 | 0.1308 | 0.6913 | 0.7639 | 0.8460 |
| OR4 | 10000 | 0.7801 | 0.0817 | 0.7230 | 0.7758 | 0.8308 |
| OR5 | 10000 | 0.8461 | 0.1322 | 0.7573 | 0.8274 | 0.9163 |
| OR6 | 10000 | 0.7771 | 0.1345 | 0.6924 | 0.7656 | 0.8515 |
| OR7 | 10000 | 0.6868 | 0.0762 | 0.6346 | 0.6859 | 0.7376 |
| OR8 | 10000 | 0.8377 | 0.1113 | 0.7626 | 0.8286 | 0.9012 |
| OR9 | 10000 | 0.7583 | 0.1104 | 0.6854 | 0.7533 | 0.8250 |
| OR10 | 10000 | 0.7402 | 0.0701 | 0.6907 | 0.7386 | 0.7864 |
| OR11 | 10000 | 0.7947 | 0.1016 | 0.7270 | 0.7854 | 0.8570 |
| OR12 | 10000 | 0.8577 | 0.1288 | 0.7692 | 0.8414 | 0.9259 |
| OR13 | 10000 | 0.7365 | 0.1201 | 0.6592 | 0.7334 | 0.8094 |
| OR14 | 10000 | 0.9382 | 0.1443 | 0.8361 | 0.9168 | 1.0183 |
| OR15 | 10000 | 0.7954 | 0.1174 | 0.7185 | 0.7849 | 0.8625 |
| OR16 | 10000 | 0.8290 | 0.1265 | 0.7470 | 0.8138 | 0.8961 |
| OR17 | 10000 | 0.8502 | 0.1424 | 0.7578 | 0.8290 | 0.9196 |
| OR18 | 10000 | 0.8082 | 0.1391 | 0.7171 | 0.7908 | 0.8819 |
| OR19 | 10000 | 0.7840 | 0.1338 | 0.6993 | 0.7748 | 0.8561 |
| OR20 | 10000 | 0.8078 | 0.1185 | 0.7279 | 0.7948 | 0.8771 |
| OR21 | 10000 | 0.7190 | 0.1049 | 0.6503 | 0.7182 | 0.7879 |
| OR22 | 10000 | 0.7064 | 0.1077 | 0.6348 | 0.7055 | 0.7753 |
| Pooled | 10000 | 0.7810 | 0.0529 | 0.7446 | 0.7795 | 0.8151 |
You can use  CATER autocall macro to create a caterpillar plot of the odds ratios. The following statement takes the output from the Nlout data set and generates a caterpillar plot of all study-specific treatment effects and the overall treatment effect:
CATER autocall macro to create a caterpillar plot of the odds ratios. The following statement takes the output from the Nlout data set and generates a caterpillar plot of all study-specific treatment effects and the overall treatment effect:
%CATER(data=blout, var=OR: Pooled);
Figure 4 shows  credible intervals of odds ratios from the 22 studies. Comparing Figure 2 and Figure 4, you can see that the posterior means and the credible intervals that are obtained by using the approximate normal likelihood and the binomial likelihood are very similar.
credible intervals of odds ratios from the 22 studies. Comparing Figure 2 and Figure 4, you can see that the posterior means and the credible intervals that are obtained by using the approximate normal likelihood and the binomial likelihood are very similar.

Figure 5 compares the kernel density plots of the odds ratios that are produced by using the approximate normal likelihood and the exact binomial likelihood. The first plot shows the comparison for the thirteenth study, and the second plot shows the comparison for the nineteenth study. Even though the observed mortality rates ( ) are close to 0 for both studies, the kernel density plots that are produced by using the approximate normal likelihood and the exact binomial likelihood are very similar. Hence, this example shows very little sensitivity to the likelihood assumption. In general, this can be explained by the large sample sizes of the studies included in the meta-analysis.
) are close to 0 for both studies, the kernel density plots that are produced by using the approximate normal likelihood and the exact binomial likelihood are very similar. Hence, this example shows very little sensitivity to the likelihood assumption. In general, this can be explained by the large sample sizes of the studies included in the meta-analysis.

References
-
Spiegelhalter, D. J., Abrams, K. R., and Myles, J. P. (2004), Bayesian Approaches to Clinical Trials and Health-Care Evaluation, Chichester, England: John Wiley & Sons.
-
Yusuf, S., Petro, R., Lewis, J., Collins, R., and Sleight, P. (1985), “Beta Blockade During and After Myocardial Infarction: An Overview of Randomized Trials,” Progress in Cardiovascular Diseases, 27, 335–371.
These sample files and code examples are provided by SAS Institute Inc. "as is" without warranty of any kind, either express or implied, including but not limited to the implied warranties of merchantability and fitness for a particular purpose. Recipients acknowledge and agree that SAS Institute shall not be liable for any damages whatsoever arising out of their use of this material. In addition, SAS Institute will provide no support for the materials contained herein.
data multistudies;
input studyid ctrl ctrlN trt trtN;
datalines;
1 3 39 3 38
2 14 116 7 114
3 11 93 5 69
4 127 1520 102 1533
5 27 365 28 355
6 6 52 4 59
7 152 939 98 945
8 48 471 60 632
9 37 282 25 278
10 188 1921 138 1916
11 52 583 64 873
12 47 266 45 263
13 16 293 9 291
14 45 883 57 858
15 31 147 25 154
16 38 213 33 207
17 12 122 28 251
18 6 154 8 151
19 3 134 6 174
20 40 218 32 209
21 43 364 27 391
22 39 674 22 680
;
data multistudies;
set multistudies;
logy=log(trt/(trtN-trt)/(ctrl/(ctrlN-ctrl)));
sigma2=1/trt+1/(trtN-trt)+1/ctrl+1/(ctrlN-ctrl);
run;
proc mcmc data=multistudies outpost=nlout seed=276 nmc=50000 thin=5
monitor=(OR Pooled);
array OR[22];
parms mu tau2;
prior mu ~ normal(0, sd=3);
prior tau2~ igamma(0.01,s=0.01);
random theta ~n(mu, var=tau2) subject=studyid;
OR[studyid]=exp(theta);
Pooled=exp(mu);
model logy ~ n(theta, var=sigma2);
run;
%CATER(data=nlout, var=OR: Pooled);
proc mcmc data=multistudies outpost=blout seed=126 nmc=50000 thin=5
monitor=(OR Pooled);
array OR[22];
parms mu_theta mu_phi s_theta s_phi;
prior mu:~ normal(0,sd=3);
prior s:~ igamma(0.01,s=0.01);
random theta ~n(mu_theta, var=s_theta) subject=studyid;
random phi ~n(mu_phi, var=s_phi) subject=studyid;
p = logistic(theta + phi);
q = logistic(phi);
model trt ~ binomial(trtN, p);
model ctrl ~ binomial(ctrlN, q);
OR[studyid]=exp(theta);
Pooled=exp(mu_theta);
run;
%CATER(data=blout, var=OR: Pooled);
data blout;
set blout;
array OR {22} OR1-OR22;
array y_{22} y_1-y_22;
keep y_1-y_22;
do i=1 to 22;
y_{i}=OR{i};
end;
run;
data all;
set nlout blout;
keep OR13 OR19 y_13 y_19;
run;
proc template;
define statgraph OneByTwo;
begingraph;
layout lattice / rows=1 columns=2 rowgutter=10 columngutter=10;
layout overlay;
densityplot OR13 / kernel() lineattrs=GRAPHDATA2 name='n' legendlabel='normal';
densityplot y_13 / kernel() lineattrs=GRAPHDATA1 name='b' legendlabel='binomial';
endlayout;
layout overlay;
densityplot OR19 / kernel()lineattrs=GRAPHDATA2 name='n' legendlabel='normal';
densityplot y_19 / kernel() lineattrs=GRAPHDATA1 name='b' legendlabel='binomial';
endlayout;
sidebar/ align=bottom;
discretelegend "n" "b"/ border=1 ;
endsidebar;
endlayout;
endgraph;
end;
run;
proc sgrender data=all template=OneByTwo;
run;
These sample files and code examples are provided by SAS Institute Inc. "as is" without warranty of any kind, either express or implied, including but not limited to the implied warranties of merchantability and fitness for a particular purpose. Recipients acknowledge and agree that SAS Institute shall not be liable for any damages whatsoever arising out of their use of this material. In addition, SAS Institute will provide no support for the materials contained herein.
| Type: | Sample |
| Topic: | Analytics ==> Bayesian Analysis |
| Date Modified: | 2017-01-12 15:22:16 |
| Date Created: | 2016-10-25 10:16:12 |
Operating System and Release Information
| Product Family | Product | Host | SAS Release | |
| Starting | Ending | |||
| SAS System | SAS/STAT | z/OS | ||
| z/OS 64-bit | ||||
| OpenVMS VAX | ||||
| Microsoft® Windows® for 64-Bit Itanium-based Systems | ||||
| Microsoft Windows Server 2003 Datacenter 64-bit Edition | ||||
| Microsoft Windows Server 2003 Enterprise 64-bit Edition | ||||
| Microsoft Windows XP 64-bit Edition | ||||
| Microsoft® Windows® for x64 | ||||
| OS/2 | ||||
| Microsoft Windows 8 Enterprise 32-bit | ||||
| Microsoft Windows 8 Enterprise x64 | ||||
| Microsoft Windows 8 Pro 32-bit | ||||
| Microsoft Windows 8 Pro x64 | ||||
| Microsoft Windows 8.1 Enterprise 32-bit | ||||
| Microsoft Windows 8.1 Enterprise x64 | ||||
| Microsoft Windows 8.1 Pro 32-bit | ||||
| Microsoft Windows 8.1 Pro x64 | ||||
| Microsoft Windows 10 | ||||
| Microsoft Windows 95/98 | ||||
| Microsoft Windows 2000 Advanced Server | ||||
| Microsoft Windows 2000 Datacenter Server | ||||
| Microsoft Windows 2000 Server | ||||
| Microsoft Windows 2000 Professional | ||||
| Microsoft Windows NT Workstation | ||||
| Microsoft Windows Server 2003 Datacenter Edition | ||||
| Microsoft Windows Server 2003 Enterprise Edition | ||||
| Microsoft Windows Server 2003 Standard Edition | ||||
| Microsoft Windows Server 2003 for x64 | ||||
| Microsoft Windows Server 2008 | ||||
| Microsoft Windows Server 2008 R2 | ||||
| Microsoft Windows Server 2008 for x64 | ||||
| Microsoft Windows Server 2012 Datacenter | ||||
| Microsoft Windows Server 2012 R2 Datacenter | ||||
| Microsoft Windows Server 2012 R2 Std | ||||
| Microsoft Windows Server 2012 Std | ||||
| Microsoft Windows XP Professional | ||||
| Windows 7 Enterprise 32 bit | ||||
| Windows 7 Enterprise x64 | ||||
| Windows 7 Home Premium 32 bit | ||||
| Windows 7 Home Premium x64 | ||||
| Windows 7 Professional 32 bit | ||||
| Windows 7 Professional x64 | ||||
| Windows 7 Ultimate 32 bit | ||||
| Windows 7 Ultimate x64 | ||||
| Windows Millennium Edition (Me) | ||||
| Windows Vista | ||||
| Windows Vista for x64 | ||||
| 64-bit Enabled AIX | ||||
| 64-bit Enabled HP-UX | ||||
| 64-bit Enabled Solaris | ||||
| ABI+ for Intel Architecture | ||||
| AIX | ||||
| HP-UX | ||||
| HP-UX IPF | ||||
| IRIX | ||||
| Linux | ||||
| Linux for x64 | ||||
| Linux on Itanium | ||||
| OpenVMS Alpha | ||||
| OpenVMS on HP Integrity | ||||
| Solaris | ||||
| Solaris for x64 | ||||
| Tru64 UNIX | ||||