The MULTTEST Procedure
References
-
Agresti, A. (2002), Categorical Data Analysis, Second Edition, New York: John Wiley & Sons.
-
Allison, D. B., Gadbury, G. L., Moonseong, H., Fernández, J. R., Lee, C., Prolla, T. A., and Weindruch, R. (2002), “A Mixture Model Approach for the Analysis of Microarray Gene Expression Data,” Computational Statistics and Data Analysis, 39, 1–20.
-
Armitage, P. (1955), “Tests for Linear Trend in Proportions and Frequencies,” Biometrics, 11, 375–386.
-
Benjamini, Y. and Hochberg, Y. (1995), “Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing,” Journal of the Royal Statistical Society, Series B, 57, 289–300.
-
Benjamini, Y. and Hochberg, Y. (2000), “On the Adaptive Control of the False Discovery Rate in Multiple Testing with Independent Statistics,” Journal of Educational and Behavioral Statistics, 25, 60–83.
-
Benjamini, Y., Krieger, A. M., and Yekutieli, D. (2006), “Adaptive Linear Step-up False Discovery Rate Controlling Procedures,” Biometrika, 93, 491–507.
-
Benjamini, Y. and Yekateuli, D. (2001), “The Control of the False Discovery Rate in Multiple Testing under Dependency,” Annals of Statistics, 29, 1165–1188.
-
Bickis, M. and Krewski, D. (1986), “Statistical Issues in the Analysis of the Long Term Carcinogenicity Bioassay in Small Rodents: An Empirical Evaluation of Statistical Decision Rules,” Environmental Health Directorate.
-
Brown, B. W. and Russell, K. (1997), “Methods Correcting for Multiple Testing: Operating Characteristics,” Statistics in Medicine, 16, 2511–2528.
-
Brown, C. C. and Fears, T. R. (1981), “Exact Significance Levels for Multiple Binomial Testing with Application to Carcinogenicity Screens,” Biometrics, 37, 763–774.
-
Cochran, W. G. (1954), “Some Methods for Strengthening the Common
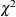 Tests,” Biometrics, 10, 417–451.
Tests,” Biometrics, 10, 417–451.
-
Dinse, G. E. (1985), “Testing for Trend in Tumor Prevalence Rates: I. Nonlethal Tumors,” Biometrics, 41, 751–770.
-
Dmitrienko, A., Molenberghs, G., Chuang-Stein, C., and Offen, W. (2005), Analysis of Clinical Trials Using SAS: A Practical Guide, Cary, NC: SAS Institute Inc.
-
Dudoit, S., Shaffer, J. P., and Boldrick, J. C. (2003), “Multiple Hypothesis Testing in Microarray Experiments,” Statistical Science, 18, 71–103.
-
Freedman, D. A. (1981), “Bootstrapping Regression Models,” Annals of Statistics, 9, 1218–1228.
-
Freeman, M. F. and Tukey, J. W. (1950), “Transformations Related to the Angular and the Square Root,” Annals of Mathematical Statistics, 21, 607–611.
-
Gibson, G. and Wolfinger, R. D. (2004), “Gene Expression Profiling Using Mixed Models,” in A. M. Saxton, ed., Genetic Analysis of Complex Traits Using SAS, 251–278, Cary, NC: SAS Publishing.
-
Good, I. J. (1987), “A Survey of the Use of the Fast Fourier Transform for Computing Distributions,” Journal of Statistical Computation and Simulation, 28, 87–93.
-
Heyse, J. and Rom, D. (1988), “Adjusting for Multiplicity of Statistical Tests in the Analysis of Carcinogenicity Studies,” Biometrical Journal, 30, 883–896.
-
Hochberg, Y. (1988), “A Sharper Bonferroni Procedure for Multiple Significance Testing,” Biometrika, 75, 800–803.
-
Hochberg, Y. and Benjamini, Y. (1990), “More Powerful Procedures for Multiple Significance Testing,” Statistics in Medicine, 9, 811–818.
-
Hochberg, Y. and Tamhane, A. C. (1987), Multiple Comparison Procedures, New York: John Wiley & Sons.
-
Hoel, D. G. and Walburg, H. E. (1972), “Statistical Analysis of Survival Experiments,” Journal of the National Cancer Institute, 49, 361–372.
-
Holland, B. S. and Copenhaver, M. D. (1987), “An Improved Sequentially Rejective Bonferroni Test Procedure,” Biometrics, 43, 417–424.
-
Holm, S. (1979), “A Simple Sequentially Rejective Multiple Test Procedure,” Scandinavian Journal of Statistics, 6, 65–70.
-
Hommel, G. (1988), “A Comparison of Two Modified Bonferroni Procedures,” Biometrika, 75, 383–386.
-
Hsueh, H., Chen, J. J., and Kodell, R. L. (2003), “Comparison of Methods for Estimating the Number of True Null Hypotheses in Multiplicity Testing,” Journal of Biopharmaceutical Statistics, 13, 675–689.
-
Lagakos, S. W. and Louis, T. A. (1985), “The Statistical Analysis of Rodent Tumorigenicity Experiments,” in D. B. Clayson, D. Krewski, and I. Munro, eds., Toxicological Risk Assessment, 144–163, Boca Raton, FL: CRC Press.
-
Liu, W. (1996), “Multiple Tests of a Non-hierarchical Finite Family of Hypotheses,” Journal of the Royal Statistical Society, Series B, 58, 455–461.
-
Mantel, N. (1980), “Assessing Laboratory Evidence for Neoplastic Activity,” Biometrics, 36, 381–399.
-
Mantel, N. and Haenszel, W. (1959), “Statistical Aspects of Analysis of Data from Retrospective Studies of Disease,” Journal of the National Cancer Institute, 22, 719–748.
-
Marcus, R., Peritz, E., and Gabriel, K. R. (1976), “On Closed Testing Procedures with Special Reference to Ordered Analysis of Variance,” Biometrika, 63, 655–660.
-
Miller, J. J. (1978), “The Inverse of the Freeman-Tukey Double Arcsine Transformation,” The American Statistician, 32, 138.
-
Osborne, J. A. (2006), “Estimating the False Discovery Rate Using SAS,” in Proceedings of the Thirty-first Annual SAS Users Group International Conference, Cary, NC: SAS Institute Inc.
-
Pagano, M. and Tritchler, D. (1983), “On Obtaining Permutation Distributions in Polynomial Time,” Journal of the American Statistical Association, 78, 435–440.
-
Peto, R., Pike, M. C., and Day, N. E. (1980), “Guidelines for Simple, Sensitive Significance Tests for Carcinogenic Effects in Long-Term Animal Experiments,” Long-Term and Short-Term Screening Assays for Carcinogens: A Critical Appraisal.
-
Press, W. H., Teukolsky, S. A., Vetterling, W. T., and Flannery, B. P. (1992), Numerical Recipes in C: The Art of Scientific Computing, Second Edition, Cambridge: Cambridge University Press.
-
Sarkar, S. K. and Chang, C.-K. (1997), “The Simes Method for Multiple Hypothesis Testing with Positively Dependent Test Statistics,” Journal of the American Statistical Association, 92, 1601–1608.
-
Satterthwaite, F. E. (1946), “An Approximate Distribution of Estimates of Variance Components,” Biometrics Bulletin, 2, 110–114.
-
Schweder, T. and Spjøtvoll, E. (1982), “Plots of P-Values to Evaluate Many Tests Simultaneously,” Biometrika, 69, 493–502.
-
Shaffer, J. P. (1986), “Modified Sequentially Rejective Multiple Test Procedures,” Journal of the American Statistical Association, 81, 826–831.
-
Šidák (1967), “Rectangular Confidence Regions for the Means of Multivariate Normal Distributions,” Journal of the American Statistical Association, 62, 626–633.
-
Simes, R. J. (1986), “An Improved Bonferroni Procedure for Multiple Tests of Significance,” Biometrika, 73, 751–754.
-
Soper, K. A. and Tonkonoh, N. (1993), “The Discrete Distribution Used for the Log-Rank Test Can Be Inaccurate,” Biometrical Journal, 35, 291–298.
-
Storey, J. D. (2002), “A Direct Approach to False Discovery Rates,” JRSS-B, 64, 479–498.
-
Storey, J. D., Taylor, J. E., and Siegmund, D. (2004), “Strong Control, Conservative Point Estimation, and Simultaneous Conservative Consistency of False Discovery Rates: A Unified Approach,” JRSS-B, 66, 187–205.
-
Storey, J. D. and Tibshirani, R. (2003), “Statistical Significance for Genomewide Studies,” in Proceedings of the National Academy of Sciences of the United States of America, volume 100, 9440–9445.
-
Turkheimer, F. E., Smith, C. B., and Schmidt, K. (2001), “Estimation of the Number of ’True’ Null Hypotheses in Multivariate Analysis of Neuroimaging Data,” NeuroImage, 13, 920–930.
-
Westfall, P. H. (2005), “Combining P Values,” in P. Armitage and T. Colton, eds., Encyclopedia of Biostatistics, Second Edition, 987–991, Chichester, UK: John Wiley & Sons.
-
Westfall, P. H. and Lin, Y. (1988), “Estimating Optimal Continuity Corrections in Run Time,” Proceedings of the Statistical Computing Section.
-
Westfall, P. H. and Soper, K. A. (1994), “Nonstandard Uses of PROC MULTTEST: Permutational Peto Tests; Permutational and Unconditional t and Binomial Tests,” in Proceedings of the Nineteenth Annual SAS Users Group International Conference, Cary, NC: SAS Institute Inc.
-
Westfall, P. H., Tobias, R. D., Rom, D., Wolfinger, R. D., and Hochberg, Y. (1999), Multiple Comparisons and Multiple Tests Using the SAS System, Cary, NC: SAS Institute Inc.
-
Westfall, P. H. and Wolfinger, R. D. (1997), “Multiple Tests with Discrete Distributions,” The American Statistician, 51, 3–8.
-
Westfall, P. H. and Wolfinger, R. D. (2000), “Closed Multiple Testing Procedures and PROC MULTTEST,” Observations.
-
Westfall, P. H. and Young, S. S. (1989), “P-Value Adjustments for Multiple Tests in Multivariate Binomial Models,” Journal of the American Statistical Association, 84, 780–786.
-
Westfall, P. J. and Young, S. S. (1993), Resampling-Based Multiple Testing, New York: John Wiley & Sons.
-
Yates, F. (1984), “Tests of Significance for
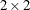 Contingency Tables,” Journal of the Royal Statistical Society.
Contingency Tables,” Journal of the Royal Statistical Society.
-
Yekateuli, D. and Benjamini, Y. (1999), “Resampling-Based False Discovery Rate Controlling Multiple Test Procedures for Correlated Test Statistics,” Journal of Statistical Planning and Inference, 82, 171–196.
