CDISC Define-XML 2.0
Purpose
The CDISC Define-XML
2.0 standard defines the metadata structures in a machine-readable
XML format. These metadata structures are used to describe tabulation
and analysis data sets and variables for regulatory submissions and
any proprietary (non-CDISC) data set structure. The XML schema that
is used to define the metadata structures in an XML format is based
on an extension to the CDISC Operational Data Model (ODM).
Release Date
CDISC Define-XML Version
2.0 specification, Production Version 2.0.0, March 5, 2013.
Regulatory Basis
(Source: CDISC Define-XML
Version 2.0 Specification)
“In the United
States, the approval process for regulated human and animal health
products requires the submission of data from clinical trials and
other studies as expressed in the Code of Federal Regulations (CFR).
The FDA established the regulatory basis for wholly electronic submission
of data in 1997 with the publication of regulations on the use of
electronic records in place of paper records (21 CFR Part 11). In
1999, the FDA standardized the submission of clinical and non-clinical
data using the SAS Version 5 XPORT Transport Format and the submission
of metadata using Portable Document Format (PDF), respectively. In
2005, the Study Data Specifications published by the FDA included
the recommendation that data definitions (metadata) be provided as
a Define-XML file. In December 2011, the CDER Common Data Standards
Issues Document stated that ‘a properly functioning define.xml
file is an important part of the submission of standardized electronic
datasets and should not be considered optional.’”
CDISC Define-XML 2.0 Reference Standard
Overview
The domain and column
metadata that constitute the SAS representation of the CDISC Define-XML
2.0 standard are derived from the global standards library in these
formats:
-
as empty data sets (using the macro %CST_CREATETABLESFORDATASTANDARD)
-
as table metadata (See CDISC Define-XML 2.0 reference_tables.)
-
as column metadata (See CDISC Define-XML 2.0 reference_columns.)
CDISC Define-XML 2.0 reference_tables
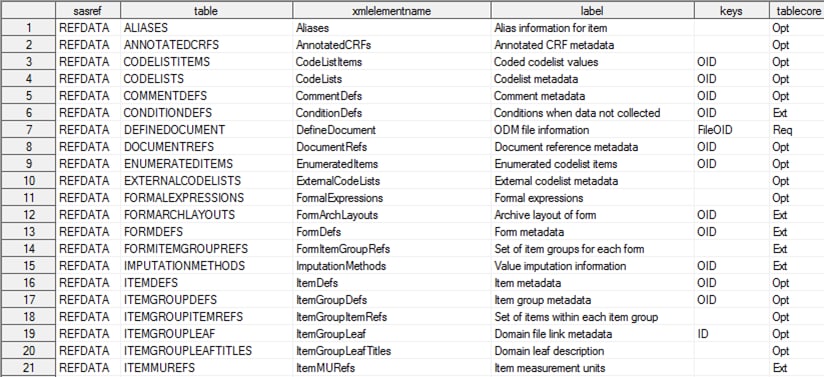
CDISC Define-XML 2.0 reference_columns
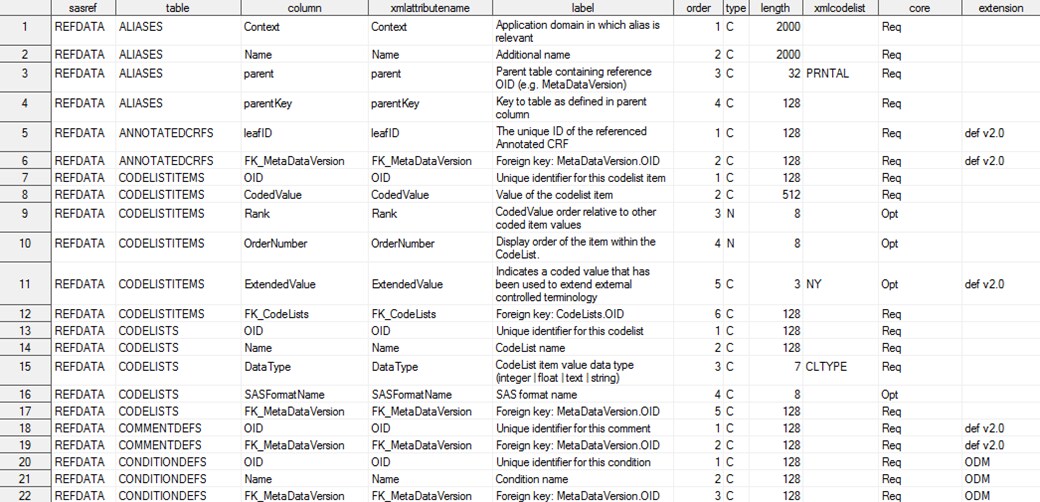
The tablecore column
in the reference_tables data set indicates whether the table is a
required (Req) or optional (Opt)
part of the Define-XML 2.0 metadata, according to the XML schema.
Tables with tablecore equal to Ext are part
of the underlying ODM metadata model, but they should be considered
extensions to the Define-XML 2.0 metadata model. The core column in
the reference_columns data set indicates whether a column is required
(Req) or optional (Opt)
in a table when the table is part of the metadata.
As a general rule, the
SAS representation of the CDISC Define-XML 2.0 standard is patterned
to match the XML element (data set) and attribute (column) structure
of define.xml. The SAS representation of the CDISC Define-XML 2.0
metadata model contains fewer tables than the CDISC Define-XML 2.0
metadata model. This reduction was accomplished by combining tables
with the same structure.
The following display
shows an example of combining tables:
CDISC Define-XML 2.0 TranslatedText Table
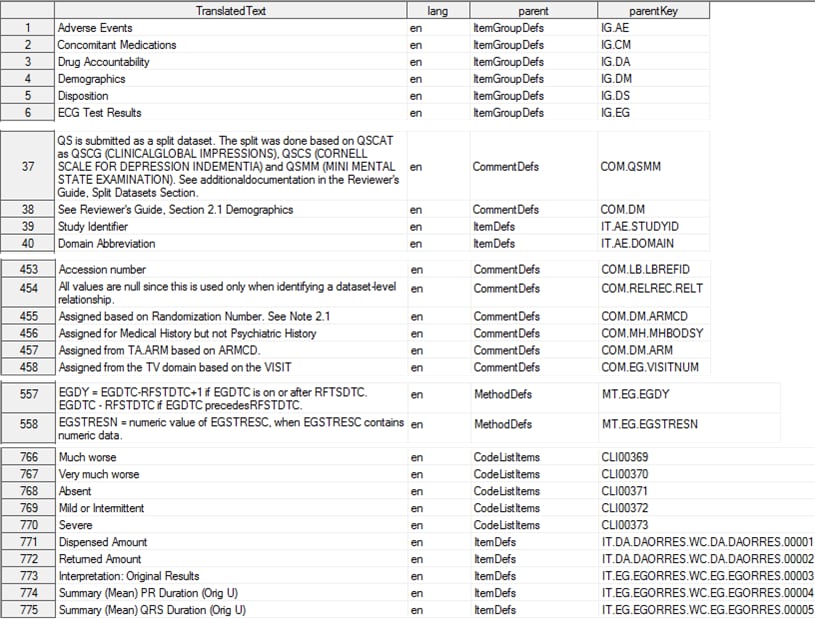
The TranslatedText table
contains the contents of the TranslatedText child elements of various
parent elements (ItemGroupDefs, ItemDefs, ItemOrigin, CodeLists, CodeListItems,
MethodDefs, CommentDefs, and others). Other tables that combine similar
table structures into one table are the Aliases table, the DocumentRefs
table, and the FormalExpressions table.
The highly structured
nature of CDISC Define-XML 2.0 data requires that any mapping to a
relational format include a large number of data sets. Foreign key
relationships help preserve the intended non-relational object structure.
In SAS Clinical Standards Toolkit, these foreign key relationships
are enforced when validating CDISC Define-XML 2.0 data sets in a way
that is similar to the CDISC CRT-DDS 1.0 data sets.
Field lengths in the
CDISC Define-XML 2.0 data sets are consistent by core data type. CDISC
has not specified a limit to the length of most character fields.
Arbitrary lengths have been chosen by data type. Here are the lengths:
|
Type Name
|
Length
|
Description
|
|---|---|---|
|
oid
|
128
|
A unique object identifier
or a reference
|
|
text
|
2000
|
A character field that
can accommodate a large number of characters
|
|
name
|
128
|
A descriptive identifier
|
|
value
|
512
|
An item of collected
or reference data
|
|
path
|
512
|
An absolute or relative
file system path or URL
|
Note: CRT-DDS 1.0 and Define-XML
2.0 use the same default lengths
In the table, standard
data types are distilled into core data types. Larger lengths have
been chosen to ensure that no data loss occurs in the SAS Clinical
Standards Toolkit pre-installed data sets. Production tables can be
compressed using SAS mechanisms to preserve disk space.
CDISC Define-XML 2.0 SAS Data Set Construction
The SAS Clinical Standards
Toolkit CDISC Define-XML 2.0 reference standard supports these actions:
-
reading and representing a define.xml file in SAS
-
building a define.xml file
-
validating the structural integrity of the define.xml file against an XML schema
To support this functionality,
supplemental files include these global standards library files:
-
A SAS format catalog (defct.sas7bcat) in the
formatsfolder provides valid values for selected columns in the 46 data sets of the SAS representation. -
The Messages data set in the
messagesfolder provides unified error messaging for all Define-XML processes. -
SAS code in the
macrosfolder provides code that is specific to CDISC Define-XML 2.0. This SAS code augments code that is provided in the primary SAS Clinical Standards Toolkit autocall library (!sasroot/cstframework/sasmacro). -
The
style sheetfolder contains the define2-0-0.xsl XSL style sheet. The define2-0-0.xsl style sheet is based on the style sheet that was published by CDISC in 2013. It can be found at http://www.cdisc.org/define-xml.A define.xml file can be rendered in a human-readable form (such as HTML) with an XSL style sheet.
Copyright © SAS Institute Inc. All Rights Reserved.