CDISC Analysis Results Metadata 1.0 for Define-XML 2.0
Purpose
The CDISC Define-XML
2.0 standard defines the metadata structures in a machine-readable
XML format. These metadata structures are used to describe tabulation
and analysis data sets and variables for regulatory submissions, as
well as any proprietary (non-CDISC) data set structure.
The Analysis Results
Metadata extension to the Define-XML 2.0.0 describes a model for the
purpose of submissions to regulatory agencies such as the United States
Food and Drug Administration (FDA) as well as for the exchange of
analysis datasets and key results between other parties. This Analysis
Results Metadata extension is based on the metadata model as described
in the CDISC ADaM Analysis Data Model Version 2.1 document.
The XML schema that
is used to define the metadata structures in an XML format is based
on an extension to the CDISC Operational Data Model (ODM).
Release Date
CDISC Analysis Results
Metadata Specification for Define-XML Version 2, Production Version
1.0, January 27, 2015.
Regulatory Basis
(Source: Technical Conformance
Guide on Electronic Study Data Submissions, Pharmaceuticals and Medical
Devices Agency, Provisional Translation [as of July 2015]).
In order for the review
of clinical study data to progress smoothly, it is important that
the relationship between the analysis results shown in the application
documents and the analysis datasets is easily understandable. Therefore,
the definition documents of the ADaM datasets should preferably include
Analysis Results Metadata, which shows the relationship between the
analysis results and the corresponding analysis dataset and the variables
used, for the analyses performed to obtain the main results of efficacy
and safety and clinical study results that provide the rationales
for setting of the dosage and administration, shown in 4.1.1.3. The
Analysis Results Metadata of each analysis should preferably include
the following items.
-
Figure or table numbers and titles showing the analysis results displayed in the clinical study report
-
Purpose and reasons for performing the analysis
-
Parameter name and code to be used
-
Variables subject to analysis
-
Dataset to be used
-
Selection criteria for the records subject to analysis
-
Corresponding description in the statistical analysis plan, analysis program name, and summary of the analytical methods
-
Extract of the analysis program corresponding to the analysis method
For the format of the
Analysis Results Metadata, the applicant should refer to the Analysis
Results Metadata Specification for Define-XML by CDISC to the extent
possible, but if it is difficult to include it into the definition
document, it is possible to submit it as a separated file in PDF format,
as specified in “Electronic Specifications of Common Technical
Documents”, and “Handling of Electronic Specifications
of Common Technical Documents”. The explanations in the definition
document may be written in Japanese.
CDISC Define-XML 2.0 Reference Standard (including Analysis Results Metadata)
The domain and column
metadata that constitute the SAS representation of the CDISC Define-XML
2.0 standard (including Analysis Results Metadata) are derived from
the global standards library in these formats:
-
as empty data sets (using the macro %CST_CREATETABLESFORDATASTANDARD)
-
as table metadata for 54 data sets (reference_tables in the standard metadata folder. For more information, see reference_tables (CDISC Define-XML 2.0 including Analysis Results Metadata).)
-
as column metadata for 239 columns in the 54 data sets (reference_columns in the standard metadata folder. For more information, see reference_columns (CDISC Define-XML 2.0 including Analysis Results Metadata).)
reference_tables (CDISC Define-XML 2.0 including Analysis Results
Metadata)
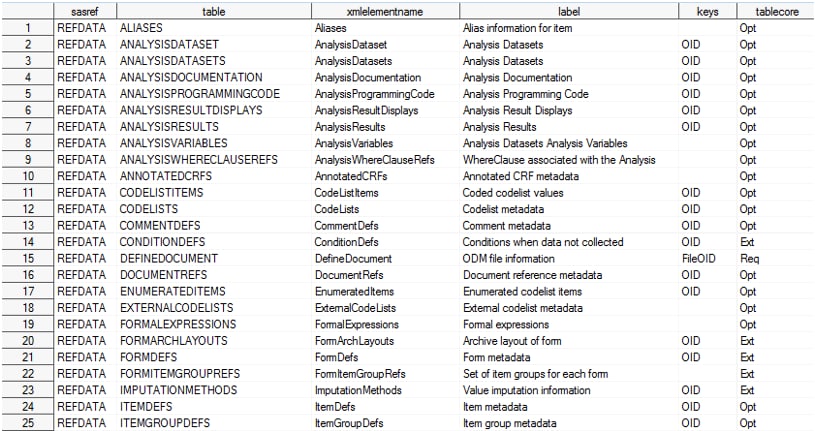
reference_columns (CDISC Define-XML 2.0 including Analysis
Results Metadata)
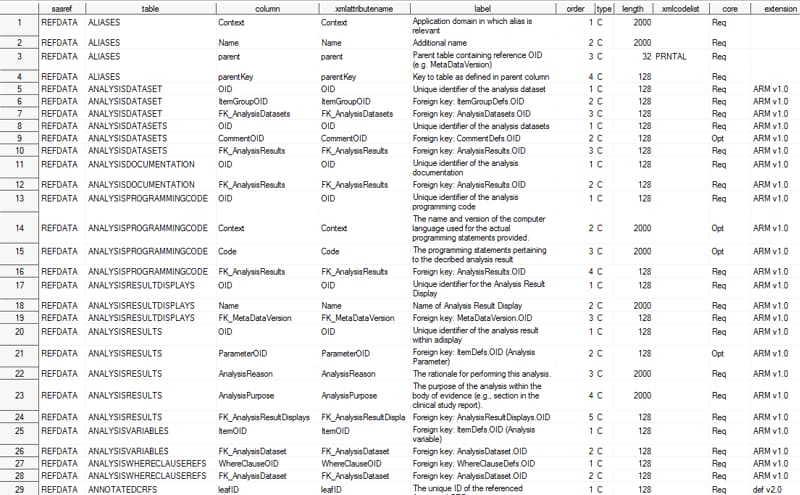
The tablecore column
in the reference_tables data set indicates whether the table is a
required (Req) or optional (Opt)
part of the Define-XML 2.0 metadata, according to the XML schema.
Tables with tablecore equal to Ext are part
of the underlying ODM metadata model, but they should be considered
extensions to the Define-XML 2.0 metadata model. The core column in
the reference_columns data set indicates whether a column is required
(Req) or optional (Opt)
in a table when the table is part of the metadata.
Copyright © SAS Institute Inc. All Rights Reserved.