Metadata Requirements
Overview
As noted in Supported Standards, a standard consists of properties, messages, and metadata
files that collectively represent the standard in the SAS Clinical
Standards Toolkit. Each SAS Clinical Standards Toolkit registered
standard can support validation if the standards.supportsvalidation
flag is set to Y. This setting indicates that the required set of
validation files defining the standard exist. By default, the set
of validation files that supports the standards that are provided
by SAS is in the cstGlobalLibrary folder hierarchy.
For example, validation
files that define the CDISC SDTM 3.1.3 standard are in this folder
hierarchy:
global standards library directory/standards/cdisc-sdtm-3.1.3–1.7The following sections
describe each metadata type used by typical validation processes. For information
about metadata files that are common to all SAS Clinical Standards
Toolkit processes, see Metadata File Descriptions. Metadata characteristics specific
to compliance assessments are described in the sections in this chapter.
Reference Metadata
For CDISC standards,
reference metadata about data sets is defined in a reference_tables
data set, and metadata about columns is defined in a reference_columns
data set. An example
of a CDISC SDTM reference_tables record is provided in reference_tables Data Set and an example
of a CDISC SDTM reference_columns record is provided in reference_columns Data Set.
Note: The structure and content
of the reference metadata data sets can vary across standards.
As noted in Supported Standards, each standard
that is provided by SAS provides a SAS interpretation of the published
source guidelines or specification of that standard. Each standard is designed to serve as a representative
model or template of the source specification. Each model or template
can be modified to establish your own gold standard.
|
Column Name
|
Column Length
|
Description
|
|---|---|---|
|
sasref
|
$8
|
The SAS libref that
refers to the table in the SAS Clinical Standards Toolkit process.
This value should match the value of the SASReferences.sasref field,
where type=referencemetadata and subtype=table. This column is required.
|
|
table
|
$32
|
The name of the tabulation
domain or analysis data set being defined in the standard. The value
must conform to SAS naming conventions. This column is required.
|
|
label
|
$200
|
The label of the domain
being defined in the standard. The value must conform to SAS naming
conventions. This column is required for standards from which define.xml
metadata is derived.
|
|
class
|
$40
|
The observation class
in the standard. Example CDISC SDTM values are Events, Findings, Interventions,
Relates, Special Purpose, and Trial Design. This column is optional
and not relevant for all standards.
|
|
xmlpath
|
$200
|
The path to the SAS
transport file. This path can be specified as a relative path. The
value can be used when creating define.xml to populate the value for
the def:leaf xlink:href link to the domain file. The value should
be the pathname and filename of the SAS transport file relative to
the location of define.xml file. This column is optional and not relevant
for all standards.
|
|
xmltitle
|
$200
|
The title of the SAS
transport file. The value can be used when creating a define.xml file
to populate the value for the def:leaf def:title value. It can provide
a meaningful description, label, or location of the domain leaf (for
example, crt/data sets/Protocol 1234/AE.xpt). This column is optional
and not relevant for all standards.
|
|
structure
|
$200
|
The description of the
general structure of the table. An example value is one record per
event per subject. This column is optional and not relevant for all
standards.
|
|
purpose
|
$20
|
The description of the
general purpose of the table. Examples are Tabulation (required for
CDISC SDTM) and Analysis (required for CDISC ADaM). This column is
optional and not relevant for all standards.
|
|
keys
|
$200
|
A space-delimited string
of keys that captures the table columns that uniquely define records
in the table. This set of keys can also define the sort order of records
in the table. Example is STUDYID USUBJID. This column is expected
to support SAS Clinical Standards Toolkit functionality but is not
required for all standards.
|
|
state
|
$20
|
A description of the
table state, such as Draft or Final. This column is optional.
|
|
date
|
$20
|
A meaningful, distinguishing
date that describes the table, such as the release date, the creation
date, or the modified date. This column is optional.
|
|
standard
|
$20
|
This value captures
the standard name. This value must match the name of a registered
standard in the SAS Clinical Standards Toolkit framework. For a discussion
of registered standards, see Framework. This value must match the standard
field in the SASReferences data set. Examples are CDISC SDTM and CDISC
CRT-DDS. This column is required.
|
|
standardversion
|
$20
|
This value captures
a specific version of a standard. This value must match one of the
standard versions associated with a registered standard. This value
must match the standardversion field in the SASReferences data set.
Examples are 3.2 and 1.0. This column is required.
|
|
standardref
|
$200
|
Any reference to an
associated standard definition, implementation guide, schema, and
so on, that provides additional information about the table or describes
the table in greater detail. This column is optional.
|
|
comment
|
$500
|
Any character string
that provides comments relevant to the table. This column is optional.
|
Note: The column length can vary
to match submission requirements or corporate conventions.
|
Column Name
|
Column Length
|
Description
|
|---|---|---|
|
sasref
|
$8
|
The SAS libref that
refers to the table containing the column in the SAS Clinical Standards
Toolkit process. This value should match the value of the SASReferences.sasref
field, where type=referencemetadata and subtype=column. This column
is required.
|
|
table
|
$32
|
The name of the tabulation
domain or analysis data set being defined in the standard. The value
must conform to SAS naming conventions. This column is required.
|
|
column
|
$32
|
The name of the column
in the table. The value must conform to SAS naming conventions. This
column is required.
|
|
label
|
$200
|
The label of the column.
The value must conform to SAS naming conventions. This column is required
for standards from which define.xml metadata is derived.
|
|
order
|
8.
|
The order of the columns
in each table. Values must be integers >0 and unique in each table.
This column is required.
|
|
type
|
$1
|
The SAS type, N for
numeric, C for character. This column is required.
|
|
length
|
8.
|
The length of the column.
Numeric columns have a length of 8. This column is required.
|
|
displayformat
|
$32
|
The display format for
numeric variables. For example, 8.2 indicates that floating-point
variable values should be displayed to the second decimal place. This
value is optional and not relevant for all standards.
|
|
xmldatatype
|
$8
|
The data type of the
column as it is defined in the define.xml file. Values are integer
| float | date | datetime | time | text. This column is optional and
not relevant for all standards.
|
|
xmlcodelist
|
$32
|
A SAS format name that
is used to assess conformance to controlled terminology. This value
does not have a $ prefix for character formats and does not have the
trailing period. This value is also the codelist name in the define.xml
file. The SAS format name must be in the format search path for successful
column-value validation. This record is optional and not relevant
for all standards.
|
|
core
|
$10
|
The value indicates
whether the column is required. Sample CDISC SDTM values are Req (required),
Exp (expected), Perm (permissible), and Dep (deprecated). This column
is optional and not relevant for all standards.
|
|
origin
|
$40
|
Information about the
source of the column. Values can include CRF page numbers and derived
or variable references. Values are user extensible. This column is
optional and not relevant for all standards.
|
|
role
|
$200
|
Space-delimited column
classification. Examples are Identifier, Topic, Qualifier, Timing,
Selection, and Analysis. Columns can have multiple roles. This column
is optional and not relevant for all standards.
|
|
term
|
$80
|
The value indicates
whether the column is subject to controlled terminology as defined
in each standard source specification. This column is optional and
not relevant for all standards.
|
|
algorithm
|
$1000
|
Imputation or computation
method to derive the column value. This column is optional and not
be relevant for all standards.
|
|
qualifiers
|
$200
|
Space-delimited string
containing supplemental column attributes. Example CDISC SDTM values
are MIXEDCASE, UPPERCASE, DATETIME, and DURATION. This column is optional
and not relevant for all standards.
|
|
standard
|
$20
|
This value captures
the standard name. This value must match the name of a registered
standard in the SAS Clinical Standards Toolkit framework. For a discussion
of registered standards, see Framework. This value must match the standard
field in the SASReferences data set. Examples are CDISC SDTM and CDISC
CRT-DDS. This column is required.
|
|
standardversion
|
$20
|
This value captures
a specific version of a standard. This value must match one of the
standard versions associated with a registered standard. This value
must match the standardversion field in the SASReferences data set.
Examples are 3.2 and 1.0. This column is required.
|
|
standardref
|
$200
|
Any reference to an
associated standard definition, implementation guide, schema, and
so on, that provides additional information about the column or describes
the column in greater detail. This column is optional.
|
|
comment
|
$1000
|
Any character string
that provides comments relevant to the column. This column is optional.
|
Note: The column length can vary
to match submission requirements or corporate conventions.
The standard reference
metadata provided with the SAS Clinical Standards Toolkit is in the
global standards library. By default, this library is located here:
global standards library directory/standards/<specific standard>/metadataFor example, for the
CDISC SDTM 3.1.3 standard, the location is:
global standards library directory/standards/cdisc-sdtm-3.1.3-1.7/metadataThis global standards
library metadata folder can contain other standard-specific metadata.
For example, CDISC SDTM includes class_tables and class_columns data
sets. These data sets have more generic metadata than specific domain
instances like DM or AE, and they are most useful when deriving new,
custom domains. For example, if a new CDISC SDTM events domain is
required, you can initialize table metadata based on the EVENTS record
in class_tables data set, and can initialize column metadata based
on the EVENTS, IDENTIFIERS, and TIMING records in the class_columns
data set.
Source Metadata
The SAS Clinical Standards
Toolkit validation processes require source metadata that describes
source (study) domains and columns. This is the study data that is
to be validated. The SAS Clinical Standards Toolkit assumes that the
reference metadata (that is, reference_tables and reference_columns)
for a standard serves as a model or template for the source metadata
(that is, source_tables and source_columns). It is recommended that
these two sets of metadata be structurally equivalent. However, additional
metadata attributes might exist if they are used for other purposes
or for custom extensions to the SAS Clinical Standards Toolkit.
The SAS Clinical Standards
Toolkit assumes that source_tables and source_columns data sets accurately
reflect and are consistent with the source data that they describe.
Although some standard-specific validation checks might look for discrepancies
and report them in detail, failure to accurately reflect and be consistent
with the source data can lead to errors in the SAS Clinical Standards
Toolkit validation process. It can even halt the execution of the
process.
Validation Check Metadata: Validation Master
The Validation Master
data set contains all validation checks defined for a standard. By
default, this data set is deployed to this directory in each supported
standard:
global standards library directory/standards/<standard>/validation/controlBy default, the Validation
Master SAS data set’s actual name is validation_master.sas7bdat.
The SAS Clinical Standards
Toolkit requires that this data set have a fixed structure.
The following table
lists the columns in the Validation Master data set:
|
Column Name
|
Column Length
|
Description
|
|---|---|---|
|
checkid
|
$8
|
Validation check ID.
The SAS Clinical Standards Toolkit has adopted a naming convention
matching each standard to be validated. The checkid values are prefixed
with an up to 4-character prefix (CDISC examples: ODM, SDTM, ADAM,
and CRT). By convention, the prefix matches the mnemonic field in
the Standards data set in
global standards library directory/metadata.
This prefix is followed by a 4-digit numeric that is unique within
the standard (for example, SDTM1234). You can use any naming convention
limited to eight characters. By default, the checkid column is the
first (primary) sort field in the Validation Master data set provided
with the SAS Clinical Standards Toolkit. Sorting by checkid is not
required. This column is required.
|
|
standard
|
$20
|
This value captures
the standard name. This value must match the name of a registered
standard in the SAS Clinical Standards Toolkit framework. For a discussion
of registered standards, see Framework. This value must match the standard
field in the SASReferences data set. Examples are CDISC SDTM and CDISC
CRT-DDS. This column is required.
|
|
standardversion
|
$20
|
This value captures
a specific version of a standard. This value must match one of the
standard versions associated with a registered standard. This value
must match the standardversion field in the SASReferences data set.
The only exception to this rule is that *** can be used to signify
that the check applies to all supported versions of the standard.
For example, 3.2, 1.0, ***. If a subsequent version of the standard
is released, then *** would be applicable if the check is valid for
the new version. This column is required.
|
|
checksource
|
$40
|
A string that identifies
the source of the check. CDISC examples include SAS, WebSDM, and CDISC.
This field can contain any user-defined value. A primary use of this
field is to subset the full set of checks in the run-time Validation
Control data set. This column is required.
|
|
sourceid
|
$8
|
A reference identifier
for this check from the checksource. In the Validation Master data
set, a SAS identifier (for example, SAS0001) is used for checks provided
with the SAS Clinical Standards Toolkit with no external source. An
example is IR5250 (WebSDM identifier). This column is optional.
|
|
checkseverity
|
$40
|
The severity as assigned
by checksource. This value is mapped to these standardized values:
Note (Low), Warning (Medium), Error (High). A value is expected, although
it is not technically required. It is used in messages and reporting.
|
|
checktype
|
$20
|
General type of check.
This value categorizes checks and helps register customized checks.
Values are user extensible and can be standard specific. A primary
use of this field is to subset the full set of checks in the run-time
Validation Control data set. Example CDISC SDTM values are:
Metadata-structural—Checks
some metadata-only property (no data access required).
ColumnValue-content:
Checks a column value or compares two column values.
Date-content: Checks
ISO 8601 compliance or compares two date values.
Multirecord-content:
Looks across multiple records in a single domain.
Multitable-content:
Looks across multiple domains.
Controlterm-content:
Assesses whether column value is consistent with controlled terminology.
This column is optional.
|
|
codesource
|
$32
|
The name of the check
macro. The name must conform to SAS naming conventions. The value
must be in the SAS autocall path. An example is %CSTCHECK_NOTUNIQUE.
This column is required.
|
|
usesourcemetadata
|
$1
|
The value indicates
whether to use source metadata rather than reference metadata. The
metadata controls the derivation of domains and column lists to be
validated, program flow, and looping. Values are Y and N (default).
This column is optional.
|
|
tablescope
|
$200
|
The value specifies
the domains to be validated by the check. The domains must exist in
either or both of the reference metadata or source metadata. The value
can be in the form:
_ALL_-DM-DS: Multiple
domains that exclude one or more specific domains that are delimited
with a -.
DM: Any single domain;
can be specified as libref.domain.
DM+AE: Multiple domains
delimited with a +.
_ALL_: Multiple DM domains
that exclude specific domains delimited with a -.
SUPP**: Wildcard to
include multiple domains.
CLASS:EVENTS: All domains
capturing event results. (This syntax specifies to use table metadata
column CLASS for EVENTS as the value-similar syntax for all other
fields and values.)
[_ALL_-DM][DM]: Bracket
syntax to define sublists for comparative purposes. In this example,
all non-DM domains are compared with the DM domain.
See the Validation Master
data set for a full set of values.
This column is required.
|
|
columnscope
|
$200
|
The value specifies
one or more space-delimited columns identified for inclusion or exclusion
in the specified check. The value can be in the form:
_ALL_: All columns (equivalent
to ** or a null value).
_NA_: Not applicable
(that is, domain-level check).
AGE: Any single column.
This value can be specified as libref.domain.column or domain.column.
ARM+ARMCD: Multiple
columns delimited with a +.
**BLFL-LBBLFL: Multiple
columns that exclude specific columns delimited with a -.
**DTC: Wildcard to include
multiple columns with ** representing the domain name.
xxx**: (For example,
AE**, where ** is a column wildcard).
[**STDTC][**ENDTC]:
Bracket syntax to define sublists for comparative purposes. In this
example, all start dates are compared with all end dates. The number
of columns in each sublist must be equivalent.
See the Validation Master
data set for a full set of values.
This column is optional.
(If null, the value is equivalent to _ALL_.)
|
|
codelogic
|
$2000
|
Check-specific code
segment that is inserted into the check macro defined in codesource
and consistent with codetype. The codelogic value enables check-level
customization and allows the reuse of more general check macros. The
field length of $2000 limits the code to short code segments, although
referencing another macro or using
%include expands
this capability. The codelogic value can use global and local macro
variables (for example, variables provided as macro input parameters
and variables set within the calling code). Examples include:
If ( . <
&_cstColumn1 <&_cstColumn2),
then _cstError=1;%include
<fileref>/* where
<fileref> can be set outside of the SAS Clinical Standards Toolkit
or in the
SASReferences control data set */The previous code is
limited to filerefs set outside of the SAS Clinical Standards Toolkit
or in the SASReferences control data set.
%sdtmcheckutil_recordlookupdata _cstProblems; set&_cstDSName; if <some
condition>;run;This column is optional.
|
|
codetype
|
8.
|
This value defines whether
to use codelogic and what type of codelogic can be used in the validation
code. Values include:
0: No codelogic used.
1: DATA step statement
level. (For example, if &_cstColumn <0 then _cstError=1.)
2: Full DATA step, PROC
SQL step, or multiple steps.
3: Calls a SAS macro
or
%include that can contain only DATA step
statement level code. (For example, codetype=1.)
4: Calls a SAS macro
or
%include that can contain only full DATA
step or PROC SQL step code. (For example, codetype=2.)
This column is required.
|
|
lookuptype
|
$20
|
This value defines the
type of information to use for value comparison to some standard.
Values include:
Metadata: Use the SAS
Clinical Standards Toolkit metadata. Specifically, use the value of
the column metadata field xmlcodelist to identify the codelist (rendered
as a SAS format).
Format: Use a SAS format
from the SAS format search path.
Dataset: Use a reference
SAS data set (for example, medDRA). There are no SAS Clinical Standards
Toolkit requirements for the structure and content of the reference
SAS data set.
<extensible>:
Other user-defined values can be used if there are explicitly referenced
in user-written code.
This column in optional.
|
|
lookupsource
|
$32
|
The specific SAS format
or file associated with lookuptype. For example:
If lookuptype is metadata,
then lookupsource should be blank. The code gets the value from the
source_columns.xmlcodelist field.
If lookuptype is format,
then lookupsource should be the SAS format and must be in the format
search path if it is specified. This value should generally match
any value in source_columns.xmlcodelist for the columns specified
in columnscope. This field allows a run-time validation check against
another format.
If lookuptype is Dataset,
then lookupsource should be the name of a SAS data set. This value
is specified as the data set name (for example, meddra) or libref.dataset.
If a value is provided without a libref, then the SAS Clinical Standards
Toolkit looks for any SASReferences type=referencecterm records for
the sasref value.
This column is optional.
|
|
standardref
|
$200
|
Any reference to an
associated standard definition, implementation guide, schema, and
so on, that provides additional information about the check or describes
the basis for the check in greater detail. This column is optional.
|
|
reportingcolumns
|
$200
|
This value includes
columns not included in columnscope for code-processing purposes and
to help resolve errors. If this value is specified, then it should
be a space-delimited list of columns in the domains specified in the
tablescope field. The values of these columns can be reported in the
Results data set. This column is optional.
|
|
checkstatus
|
8.
|
This value determines
whether the check is ready to be used and included in any Validation
Control run-time data set. If the check is ready, then the value should
be set to any positive integer. Values include:
0: (inactive, default)
>0: (active)
-1: (deprecated, archived)
-2: (not implemented
in this SAS Clinical Standards Toolkit release)
This column is optional,
although it is expected.
|
|
reportall
|
$1
|
This value enables more
concise reporting of errors. Values include:
Y: (yes, report all
records, default)
N: (no)
This column is required
although not all check macro modules support abbreviated (N) reporting.
|
|
uniqueid
|
$48
|
This value provides
a unique ID for the check. It ensures uniqueness in the data set and
in the SAS Clinical Standards Toolkit. This value allows any provided
or derived check to be uniquely identifiable over time. An example
is SDTM000401CST160SDTM3202014-01-07T16:03:51CST.
Legend:
characters 1-8: checkid
characters 9-10: checkid
repeat indicator (00 unless multiple invocations of checkid are included)
characters 11-16: the
version of the SAS Clinical Standards Toolkit where the check metadata
was last materially modified
characters 17-23: standard
version
characters 24-42: implementation
datetime of the last metadata update
characters 43-48: assigning
authority
This column is optional,
although it is expected.
|
|
comment
|
$200
|
Any character string
that provides comments relevant to the check. This column is optional.
|
The content of the Validation
Master data set is based on a combination of compliance requirements
and the SAS representation of the standard.
The following table
describes a sample Validation Master data set record for the CDISC
SDTM 3.1.3 standard:
|
Column Name
|
Column Value
|
Comment
|
|---|---|---|
|
checkid
|
SDTM0860
|
The SAS Clinical
Standards Toolkit check identifier used in validation results and
reports.
|
|
standard
|
CDISC-SDTM
|
The registered standard.
|
|
standardversion
|
3.1.2
|
The standard version.
A value of *** indicates that the check is applicable to all versions
of the standard. 3.1.2 indicates it is applicable for all SDTM versions
3.1.2 and later.
|
|
checksource
|
WebSDM
|
This check originated
as a WebSDM check.
|
|
sourceid
|
R5132
|
WebSDM check R5132.
|
|
checkseverity
|
Warning
|
|
|
checktype
|
Column
|
|
|
codesource
|
cstcheck_column
|
This check uses the
%CSTCHECK_COLUMN check macro in the SAS Clinical Standards Toolkit
autocall library.
|
|
usesourcemetadata
|
Y
|
This check is run on
source data domains.
|
|
tablescope
|
RELREC
|
This check is run on
the RELREC domain.
|
|
columnscope
|
RELTYPE
|
This check evaluates
only the RELTYPE column values.
|
|
codelogic
|
if (upcase(&_cstColumn) not in
("","ONE","MANY"))
then _cstError=1; |
This logic is used in
cstcheck_column. Errors are documented in a work._cstproblems data
set.
|
|
codetype
|
1
|
This code logic is used
in the DATA step.
|
|
lookuptype
|
||
|
lookupsource
|
||
|
standardref
|
||
|
reportingcolumns
|
||
|
checkstatus
|
1
|
|
|
reportall
|
Y
|
This check reports all
errors that are identified.
|
|
uniqueid
|
SDTM086001CST150SDTM3122012-06-08T10:49:21CST
|
|
|
comment
|
The Validation Master
data set contains all validation checks for a standard, whereas the
Validation Control data set is the run-time equivalent and contains
just the validation checks to be run in a validation process. The
Validation Control data set is structurally equivalent to the Validation
Master data set. For additional
information about how the validation check metadata in the Validation
Control data set is used in the SAS Clinical Standards Toolkit validation
processes, see Special Topic: How the SAS Clinical Standards Toolkit Interprets Validation Check
Metadata.
Supplemental Validation Check Metadata: Validation Standard References
The validation standard
references data set contains additional information about each of
the checks in the Validation Master data set. This data set is used
in the validation metadata reporting process to provide additional
information to you about the origin of the check. It also provides
any supporting documentation about the check. By default, this data
set is deployed to this directory in each supported standard:
global standards library directory/standards/<standard>/validation/control|
Column Name
|
Column Length
|
Description
|
|---|---|---|
|
checkid
|
$8
|
The validation check
ID, as specified in the Validation Master data set. (See Column Descriptions of the Validation Master Data Set.)
|
|
standard
|
$20
|
This value captures
the standard name. This value must match the standard in the associated
Validation Master data set. This column is required.
|
|
standardversion
|
$20
|
This value captures
a specific version of a standard. This value should be the version
for which the supplemental reference information is applicable. This
column is required.
|
|
informationsource
|
$80
|
This value captures
the origin of the reference information. The value can be an implementation
guide, website, harmonization document, and so on. It can be any source
that can be referenced that provides insight into the check.
|
|
sourcelocation
|
$200
|
This value contains
the location in the information source, such as a page number or a
section number.
|
|
seqno
|
8.
|
This value provides
a sequence number for checkid if multiple sources of information are
available for a check. This column is required.
|
|
sourcetext
|
$2000
|
This value captures
descriptive information from the source that supports the check. This
information attempts to provide a basis for inclusion of the check.
|
The content of the Validation_StdRef
data set is based on information from any source that supports the
check.
The following table
describes information about a specific check in the Validation_StdRef
data set (record 1) for the CDISC SDTM 3.1.3 standard:
|
Column Name
|
Column Value
|
Comment
|
|---|---|---|
|
checkid
|
SDTM0860
|
The SAS Clinical Standards
Toolkit check identifier used in results and reports.
|
|
standard
|
CDISC-SDTM
|
The registered standard.
|
|
standardversion
|
3.1.2
|
The standard version.
|
|
informationsource
|
SDTM 3.1.2
Implementation Guide
|
This reference information
originated from the SDTM 3.1.2 Implementation Guide.
|
|
sourcelocation
|
3.2.2, page 20
|
Section 3.2.2, page
20 of the SDTM 3.1.2 Implementation Guide.
|
|
seqno
|
1
|
The first record for
this checkid.
|
|
sourcetext
|
Conformance with the
SDTMIG Domain Models is minimally indicated by: Following SDTM-specified
controlled terminology and format guidelines for variables, when provided
|
The text of the information
retrieved from section 3.2.2, page 20 of the SDTM 3.1.2
Implementation Guide.
|
The following table
describes information about a specific check in the Validation_StdRef
data set (record 2) for the CDISC SDTM 3.1.3 standard:
|
Column Name
|
Column Value
|
Comment
|
|---|---|---|
|
checkid
|
SDTM0860
|
The SAS Clinical Standards
Toolkit check identifier used in results and reports.
|
|
standard
|
CDISC-SDTM
|
The registered standard.
|
|
standardversion
|
3.1.2
|
The standard version.
|
|
informationsource
|
SDTM 3.1.2
Implementation Guide
|
This reference information
originated from the SDTM 3.1.2 Implementation Guide.
|
|
sourcelocation
|
Convention
|
Section 6.3.7, page
153 of the SDTM 3.1.2 Implementation Guide.
|
|
seqno
|
2
|
The second record for
this checkid.
|
|
sourcetext
|
[RELTYPE] Controlled
Terms, Codelist or Format: ONE, MANY
|
The text of the information
retrieved from section 6.3.7, page 153 of the SDTM 3.1.2
Implementation Guide.
|
Supplemental Validation Check Metadata: CDISC SDTM Domains by Check
The SAS Clinical Standards
Toolkit validation metadata, as specified in the Validation Master
data set, uses the tablescope and columnscope columns to define the
scope of the check. The scope being what domains (tables) and what
columns to validate when the check is run. The SAS Clinical Standards
Toolkit uses a shorthand syntax in these columns that is interpreted
by the SAS Clinical Standards Toolkit framework macros to build a
list of target tables and columns. For more information,
see Special Topic: How the SAS Clinical Standards Toolkit Interprets Validation Check
Metadata. The Validation_DomainsByCheck data set is located here:
global standards library directory/standards/cdisc-sdtm-3.1.x/validation/controlIt contains records
for each domain to be validated by each check in the Validation Master
data set. This data set is used by reporting tools that are provided
with the SAS Clinical Standards Toolkit to report domain-specific
errors. For more information, see Reporting. It is also available to other programs
and applications that might need to subset checks that are applicable
to specific domains.
The SDTM version of
the Validation_DomainsByCheck data set that is provided by SAS is
built from the version of the Validation Master data set that is also
provided by SAS. If the tableScope and columnScope columns are modified,
then the Validation_DomainsByCheck data set must also be modified
or rebuilt.
|
Column Name
|
Column Length
|
Description
|
|---|---|---|
|
checkid
|
$8
|
The validation check
ID, as specified in the Validation Master data set. (See Column Descriptions of the Validation Master Data Set.)
|
|
table
|
$32
|
This value captures
the domain or table name. This column is required.
|
|
standardversion
|
$20
|
This value captures
a specific version of a standard. This value must match standardversion
in the associated Validation Master data set.
|
|
checksource
|
$40
|
A string that identifies
the source of the check. This value must match checksource in the
associated Validation Master data set.
|
|
resultseq
|
8.
|
The unique invocation
of a check within the Validation Master data set. This value is incremented
if multiple record or domain combinations exist.
|
For CDISC SDTM 3.1.3
validation check SDTM0860, the Validation_DomainsByCheck data set
contains a record only for the RELREC domain because the tableScope
for this check is only RELREC. However, the SDTM0606 check looks
for non-numeric values in all tables (tableScope=_ALL_). Based on
the sample study provided by SAS, 36 records (domains) are included
in the Validation_DomainsByCheck data set for SDTM0606.
Supplemental Validation Check Metadata: CDISC ADaM Class by Check
For CDISC ADaM, the
supplemental data set is called Validation_ClassByCheck. It is located
here:
global standards library directory/standards/cdisc-adam-2.1-1.7/validation/control.
This data set
is patterned after the data set that is described in Column Descriptions of the Validation_DomainsByCheck Data Set. However, the column class ($40, Observation Class within
Standard) has been added. This addition accommodates the different
way that the ADaM reference standard is defined. For example, the
reference_tables data set, located in
/standards/cdisc-adam-2.1-1.7/metadata,
includes a BDS record that serves as a class template for all specific
implementations of BDS that are required for a study. The SAS Clinical
Standards Toolkit does not know each of the specific analysis data
sets, so the Validation_ClassByCheck data set includes records by
class, not by domain, for each check in the ADaM Validation Master
data set.
Validation.Properties
Properties specific
to validation processes are provided with the SAS Clinical Standards
Toolkit. These properties enable you to specify how validation checks
are to be processed and whether metrics are to be reported.
As with all SAS Clinical
Standards Toolkit properties files, a call to the %CST_SETPROPERTIES
macro is required to translate the properties into SAS global macro
variables. This call can be explicitly made as a driver program setup
task, or it can be made by including the Validation.Properties file
as a record in the SASReferences data set. For all standards that
support validation, the Validation.Properties file is required, even
if no metrics are wanted because the SAS Clinical Standards Toolkit
validation process does expect, and uses, the metrics global macro
variables.
The following table
describes the properties in the Validation.Properties file:
|
Property Name
|
Description
|
|---|---|
|
_cstCheckSortOrder
|
This property determines
the order in which validation checks are processed. If no value is
provided, or the default value _DATA_ is used, then the data set order
is assumed. Or, _cstCheckSortOrder can be set to sort the Validation
Control data set at run time by any fields in that data set (for example,
CHECKSOURCE CHECKID).
|
|
_cstMetrics
|
This property determines
whether to calculate and report metrics. An example value is 1=Yes.
|
|
_cstMetricsDS
|
This property sets the
SAS data set name to use to accumulate metrics during the process.
The default value is work._cstmetrics.
|
|
_cstMetricsNumSubj
_cstMetricsCntNumSubj
|
This property determines
whether to calculate and report subject-level counts. An example value
is 1=Yes, initialize _cstMetricsCntNumSubj to 0. The calculation of
subject-level counts might not be appropriate for all check macros.
|
|
_cstMetricsNumRecs
_cstMetricsCntNumRecs
|
This property determines
whether to calculate and report record-level counts. An example value
is 1=Yes, initialize cstMetricsCntNumRecs to 0.
|
|
_cstMetricsNumChecks
_cstMetricsCntNumChecks
|
This property determines
whether to summarize and report the number of checks run. An example
value is 1=Yes, initialize cstMetricsCntNumChecks to 0.
|
|
_cstMetricsNumBadChecks
_cstMetricsCntNumBadChecks
|
This property determines
whether to summarize and report the number of check invocations that
failed. An example is 1=Yes, initialize cstMetricsCntNumBadChecks
to 0.
|
|
_cstMetricsNumErrors
_cstMetricsCntNumErrors
|
This property determines
whether to summarize and report the total number of errors (resultseverity=Error)
found. An example is 1=Yes, initialize cstMetricsCntNumErrors to 0.
|
|
_cstMetricsNumWarnings
_cstMetricsCntNumWarnings
|
This property determines
whether to summarize and report the total number of warnings (resultseverity=Warning)
found. An example is 1=Yes, initialize cstMetricsCntNumWarnings to
0.
|
|
_cstMetricsNumNotes
_cstMetricsCntNumNotes
|
This property determines
whether to summarize and report the total number of notes (resultseverity=Note)
found. An example value is 1=Yes, initialize cstMetricsCntNumNotes
to 0.
|
|
_cstMetricsNumStructural
_cstMetricsCntNumStructural
|
This property determines
whether to summarize and report the total number of structural (metadata)
errors found. An example value is 1=Yes, initialize cstMetricsCntNumStructural
to 0.
|
|
_cstMetricsNumContent
_cstMetricsCntNumContent
|
This property determines
whether to summarize and report the total number of content (data)
errors found. An example value is 1=Yes, initialize cstMetricsCntNumContent
to 0.
|
|
_cstMetricsTimer
|
This property determines
whether to report the elapsed time for each check invocation. An example
value is 1=Yes.
|
By default, for all
standards that support validation, Validation.Properties is located
here:
global standards library directory/standards/<standard>/programsProperties can logically
be associated with each study. Using the CDISC SDTM 3.1.3 sample study
provided with the SAS Clinical Standards Toolkit as an example, a
study-specific instance of the Validation.Properties file is located
here:
sample study library directory/cdisc-sdtm-3.1.3–1.7.
Messages
Each SAS Clinical Standards
Toolkit registered standard that supports validation has a Validation
Master data set, and an associated Messages data set. The Validation
Master data set provides the super-set of checks defined for that
standard. The Messages data set provides messages to be generated
during the execution of each validation process. A distinct Messages
data set record is expected for each set of checkid and checksource
values in the Validation Master data set. Messages can be parameterized
and internationalized.
By default, the standard-specific
Messages data set is deployed to this directory in each supported
standard:
global standards library directory/standards/<standard>/messagesAll Messages data sets
in the SAS Clinical Standards Toolkit should have the same structure. The structure
is defined in Metadata File Descriptions.
During a process, the
SAS Clinical Standards Toolkit appends any standard-specific messages
that are required by the process to any generic SAS Clinical Standards
Toolkit framework messages that are available to all processes. This
appended Messages data set follows the naming convention that is defined
within the global macro variable _cstMessages.
Validation Metrics
Generating the SAS Clinical
Standards Toolkit validation metrics provides a meaningful denominator
for most validation checks. This enables you to more accurately assess
the relative scope of errors that are detected. Generally, the calculated
denominator is a count of the number of records processed in a domain.
This code segment, which
is extracted from a validation check macro, shows a typical calculation
of the number of records in a domain. It also shows the macro call
to add the count to the Validation Metrics data set:
data _null_;
if 0 then set &_cstDSName nobs=_numobs;
call symputx('_cstMetricsCntNumRecs',_numobs);
stop;
run;
* Write applicable metrics *;
%if &_cstMetrics %then %do;
%if &_cstMetricsNumRecs %then
%cstutil_writemetric(
_cstMetricParameter=# of records tested,
_cstResultID=&_cstCheckID,
_cstResultSeqParm=&_cstResultSeq,
_cstMetricCnt=&_cstMetricsCntNumRecs,
_cstSrcDataParm=&_cstDSname
);
%end;
Because a check can
evaluate multiple columns in a domain, the count will be greater.
In addition, a metadata-level check that does not access the domain
data directly might report the number of metadata records instead.
Metrics processing is
enabled based on settings in the Validation.Properties file. See Properties in the Validation.Properties File.
The following table
provides a description of the Validation Metrics data set, including
the meaning of each field:
|
Column Name
|
Column Length
|
Description
|
|---|---|---|
|
metricparameter
|
$40
|
A descriptive text string
that specifies the metric of interest. This string is hardcoded in
the check macro and cannot be modified without code changes. Values
should be non-null.
|
|
reccount
|
8.
|
A count of the number
of records specific to the combination of metricparameter and resultid.
This number is derived in the check macro and cannot be modified without
code changes. This column can contain a summary count of records written
to the Results data set (resultid=METRICS). Reccount can be null for
selected metricparameters, such as the assessment of elapsed time
for each check.
|
|
resultid
|
$8
|
The resultid is either
the checkid or a hardcoded constant such as METRICS. The SAS Clinical
Standards Toolkit has adopted a naming convention matching each standard.
The checkid (resultid) values are prefixed with an up to 4-character
prefix (CST for framework messaging; CDISC examples: ODM, SDTM, ADAM,
and CRT). By convention, the prefix matches the mnemonic field in
the Standards data set in
global standards library directory/metadata.
This prefix is followed by a 4-digit numeric that is unique within
the standard (for example, SDTM1234). You can use any naming convention
limited to eight characters. Values should be non-null.
|
|
srcdata
|
$200
|
The string that specifies
the domain or check macro to which the metricparameter applies. Values
should be non-null.
|
|
resultseq
|
8.
|
A counter that indicates
the record number in checkid in the Validation Control run-time set
of checks. If set to 1, then this counter is incremented only with
each repeat invocation of a check. This value enables you to link
to the Validation Control and Results data sets. Values should be
non-null.
|
The following display
shows the Validation Metrics output from a SAS Clinical Standards
Toolkit validation process running CDISC SDTM validation. The Validation
Control data set contains 11 validation checks.
Sample Validation Metrics Data Set
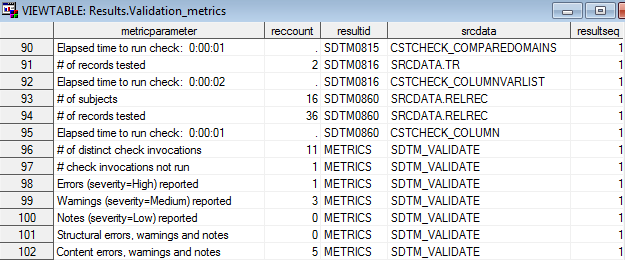
The missing reccount
value in line 90 and the absence of other metrics for SDTM0815 indicate
that the check was not run. (SDTM0815 evaluates the value of the POOLID
column, which is not used in any non-POOLDEF domain in the sample
study provided by SAS.) This should be reported in the Results data
set.
Lines 93 through 95
report metrics on the SDTM0860 validation check. Two problems are
reported in the Results data set for a single subject, and these metrics
(16 subjects and 36 records tested) provide denominator information
to assess how common the problems are.
Lines 96 through 102
are summary metrics reported at the end of the SDTM validation process
in the %SDTM_VALIDATE macro. The following five problems are noted:
-
one check (SDTM0815) could not be run
-
two of the three warnings were for SDTM0860
-
one other warning and one error condition were found
The Validation Results
and Validation Metrics data sets, when used in tandem, provide a more
complete picture of each compliance assessment.
For more information
about the Validation Metrics data set, see Column Descriptions of the Validation Metrics Data Set.
Copyright © SAS Institute Inc. All Rights Reserved.