Sample Reporting Methodology
Overview
The primary purpose
of the CDISC ADaM standard is to build analysis data sets that support
analysis and reporting of clinical research. This purpose, in turn,
supports the greater goal of submitting clinical research results
to regulatory authorities. These regulatory authorities determine
the efficacy and safety of a medical device or product.
The Analysis
Data Model (ADaM), Version 2.1 document provides specifications
for the structure and content of analysis data sets, and a suggested
metadata format for documenting the analysis results generated. Analysis
results metadata describe the major attributes of a specified analysis
result found in a clinical study report or submission. Analysis results
metadata support traceability from an analysis result used in a statistical
display to the data in the analysis data sets.
The SAS Clinical Standards
Toolkit representation of the ADaM standard includes a sample implementation
of an analysis reporting methodology.
Note: This methodology is for illustrative
purposes only. Each organization has its own set of processes and
workflows that support the generation of a clinical study report or
submission. The sample reporting methodology provided with the SAS
Clinical Standards Toolkit is intended to be representative of similar
industry reporting methodologies. The intent is not to provide a definitive
reporting methodology, but to illustrate the interaction of reporting
components through the adoption of the ADaM standard. The format for
the analysis results metadata in the SAS Clinical Standards Toolkit
has been updated for the processes that create a Define-XML 2.0 file
that include analysis results metadata according to the Analysis Results
Metadata 1.0 for Define-XML 2.0 specification.
Key clinical trial
reporting components are shown in the following table:
|
Reporting Component
|
Comments
|
|---|---|
|
Clinical Protocol, Statistical
Analysis Plan
|
Used to identify and
define data to be collected, analysis methods and algorithms to be
used, and efficacy endpoints and safety measures that determine report
output.
|
|
Source Data
|
Source data for analysis
data sets, often SDTM. Traceability back to source data is a key
ADaM requirement.
|
|
Source Metadata
|
Metadata about the source
data.
|
|
Controlled Terminology
|
Set of allowable terms
used in any source or analysis data set. For CDISC, NCI EVS serves
as the primary source of terms.
|
|
Analysis Data Sets
|
ADaM data sets, typically
including the ADSL data set and any number of BDS data sets (for example,
ADAE and ADLB) required to support analyses.
|
|
Analysis Data Set Metadata
|
Metadata about the analysis
data sets.
|
|
Analysis Results (tables,
listings, and figures)
For more information,
see Analysis Results (Tables, Listings, and Figures).
|
The set of statistical
displays (for example, text, tabular, or graphical presentation of
results) or inferential statements (such as p-values or estimates
of treatment effect).
|
|
TLF Metadata (to include
table shells)
For more information,
see TLF Metadata.
|
Commonly provided as
table shells. Can also include display-specific metadata (often as
Microsoft Excel files) used by the analysis programs to generate the
displays.
|
|
Analysis Results Metadata
For more information,
see Analysis Results Metadata.
|
Defined by the Analysis
Data Model (ADaM), Version 2.1 document, Section 5.3. For more information,
see Analysis Results Metadata.
|
|
Analysis Programs
For more information,
see Analysis Programs.
|
Programming code that
uses the analysis data sets (and, optionally, TLF metadata) to create
the analysis results.
|
|
Submission Package (for
example, eCTD)
|
The structured submission
used to package data, metadata, code, and results in a standard form
to facilitate review.
|
|
Define.xml
|
A metadata format that
documents each tabulation (SDTM) or analysis (ADaM) data set, ancillary
documents, and controlled terminology for a study or submission.
|
|
CSR/ISS/ISE
|
The focus of each ADaM
implementation. Most commonly a Clinical Study Report (CSR) for a
single clinical study. Can be an Integrated Summary of Safety (ISS)
or Integrated Summary of Efficacy (ISE) across multiple clinical studies.
|
The majority of the
files supporting the ADaM sample reporting methodology provided with
the SAS Clinical Standards Toolkit are located in the ADaM analysis
folder:
sample study library directory/cdisc-adam-2.1/sascstdemodata/analysisHere is an illustration
of the ADaM analysis folder hierarchy:
SAS Clinical Standards Toolkit ADaM Analysis Folder Hierarchy
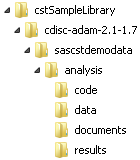
Here are noteworthy
folders:
-
The code folder contains the code to create each statistical display. This corresponds to the Analysis Results component described in Key Clinical Trial Reporting Components.
-
The data folder contains the display-specific metadata noted in the TLF Metadata component of Key Clinical Trial Reporting Components.
-
The documents folder contains table shells for the TLF Metadata component. For more information about table shells, see TLF Metadata.
-
The results folder contains several sample statistical displays, which correspond to the Analysis Results component.
TLF Metadata
A common industry reporting
strategy is to create table
shells (templates) that specify the output for each
statistical display. The SAS Clinical Standards Toolkit provides sample
table shells in this file:
sample study library directory/cdisc-adam-2.1–1.7/sascstdemodata/analysis/documents/Mock_tables_shells.pdf.
One of these displays,
a table reporting patient demographics (Table 14.2.01), follows:
SAS Clinical Standards Toolkit Sample Table Shell
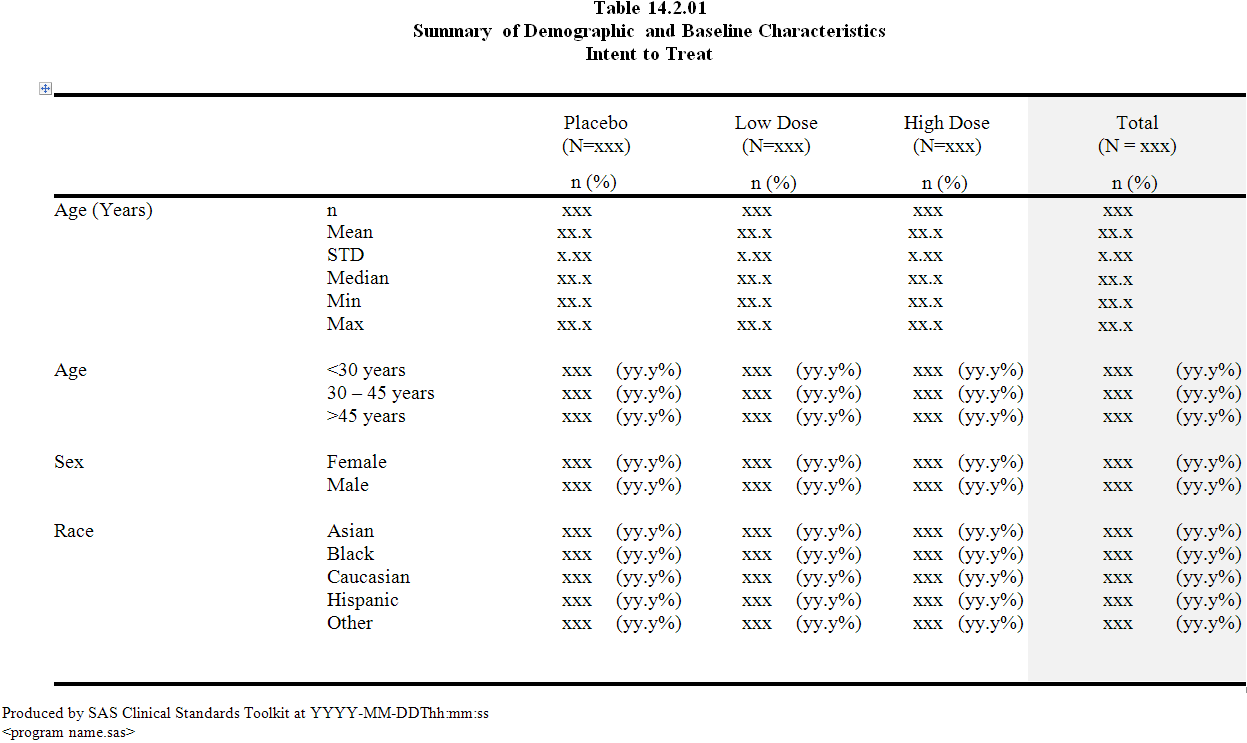
The elements of each
table shell (for example, titles, footnotes, headings, column and
row labels, cell formatting, and so on) are sometimes captured in
a metadata format, often in Microsoft Excel files. The usual intent
is to create reporting macros that can generate analysis reports based
on this metadata, so that changes in metadata are all that is required
to modify and rerun any report.
For the SAS Clinical
Standards Toolkit, sample metadata is included that demonstrates the
use of such metadata within the ADaM reporting environment.
Note: The sample metadata provided
does not represent a full implementation. All metadata fields used
in the report examples are not provided.
Supplemental metadata
is provided in this file:
sample study library directory/cdisc-adam-2.1–1.7/sascstdemodata/metadata/tlfddt.xmlTo interpret this metadata,
a sample SAS XML map file (tlfddt.map) is provided in the same folder.
SAS data sets, representing this XML metadata, are provided in the
library of SAS files located here:
sample study library directory/cdisc-adam-2.1–1.7/sascstdemodata/analysis/dataThe following figures
provide examples of some of the metadata available in the source XML
file. This metadata has been extracted into SAS data sets.
Sample TLF Metadata: Tlf_index
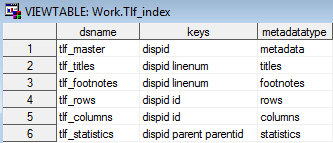
Sample TLF Metadata: Tlf_master
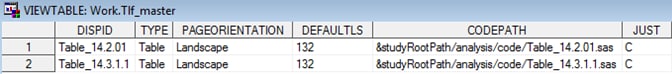
Sample TLF Metadata: Tlf_titles
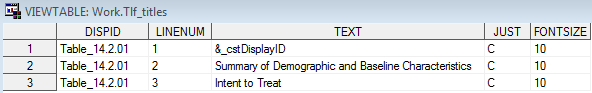
Row 1 of the Tlf_master
data set describes a centered landscape table and shows where the
generating code can be found. The title for that table is provided
in the Tlf_titles file. These tables correspond
to the table shell titles specified in SAS Clinical Standards Toolkit Sample Table Shell.
Analysis Programs
The analysis program
to generate sample Table 14.2.01 is located here:
sample study library directory/cdisc-adam-2.1–1.7/sascstdemodata/analysis/codeTwo versions are provided:
-
Table_14.2.01.sas uses the TLF metadata described previously.
-
Table_14.2.01_nomd.sas does not rely on TLF metadata to generate the report output.
As noted above, these
sample analysis programs do not fully use the sample TLF metadata
provided with the SAS Clinical Standards Toolkit. The basic coding
strategy adopted with each SAS Clinical Standards Toolkit sample analysis
program is to build each section (one or more row combinations) and
to concatenate these sections into a single input file used by PROC
REPORT.
A sample driver program
is provided to perform the process setup, to define (or reference)
the SASReferences data set, to perform any required report setup,
and to call the generic ADaM reporting macro %ADAM_CREATEDISPLAY.
This sample driver program is located here:
sample study library directory/cdisc-adam-2.1–1.7/sascstdemodata/programs/analyze_data.sasIn the sample driver
program, a call is made to %ADAM_CREATEDISPLAY for each analysis report
to be produced:
%adam_createdisplay (displaysrc=Metadata,useanalysisresults=N,usetlfddt=Y, displayid=%str(Table_14.2.01));
To automate this process
of creating all analysis reports for a study, it would be necessary
to cycle through any available metadata (such as that described
in Sample TLF Metadata: Tlf_master)
to construct multiple calls to the %ADAM_CREATEDISPLAY macro. The
%ADAM_CREATEDISPLAY macro header provides an overview of the macro
functionality and a summary of the defined macro parameters:
adam_createdisplay
Creates an analysis result display from ADaM analysis data sets.
The path to the code to create the display is provided either directly in the
macro parameters or is derived from a metadata source. Examples of metadata
sources are analysis results metadata or Tables, Listings, and Figures data
definition metadata (TLFDDT) that you maintain and reference in the
SASReferences data set.
Two primary paths (parameter settings) are supported:
1. A code source is specified. A fully qualified path is required. The
expectation is that this module is %included below to generate an
analysis result (display).
2. Metadata provides the information necessary to generate an analysis
result (display). This metadata is in the form of the CDISC ADaM
analysis results metadata, supplemental Tables, Listings, and Figures
data definition metadata (TLFDDT), or both.
@macvar studyRootPath Root path to the sample source study
@macvar _cstCTDescription Description of controlled terminology packet
@macvar _cstDebug Turns debugging on or off for the session
@macvar cstDefaultReportFormat Specifies the SAS ODS report destination
@macvar _cstGRoot Root path of the Toolkit Global Library
@macvar _cstResultsDS Results data set
@macvar _cstResultSeq Results: Unique invocation of check
@macvar _cstSASRefs Run-time SASReferences data set derived in process setup
@macvar _cstSeqCnt Results: Sequence number within _cstResultSeq
@macvar _cstSrcData Results: Source entity being evaluated
@macvar _cstStandard Name of a standard registered to Toolkit
@macvar _cstStandardVersion Version of the standard referenced in _cstStandard
@macvar _cst_rc Task error status
@macvar _cstVersion Version of the SAS Clinical Standards Toolkit
@macvar _CSTTLF_MASTERCODEPATH Dynamically derived code segment path from
TLF metadata.
@macvar workpath Path to the SAS session work library
@param _cstDisplaySrc - required - Where information comes from to generate
the result.
Values: Code | Metadata
Default: Metadata
@param _cstDisplayCode - conditional - Either a valid filename or the fully
qualified path to code that produces an analysis result. If
_cstDisplaySrc=Code, this parameter is used and is required. All of
the remaining parameters are ignored.
@param _cstUseAnalysisResults - conditional - The study-specific analysis
results metadata are used to provide report metadata.
If _cstDisplaySrc=Metadata, either this parameter or _cstUseTLFddt
must be set to Y. If both _cstUseAnalysisResults and _cstUseTLFddt
are set to Y, _cstUseAnalysisResults takes precedence.
Values: N | Y
Default: Y
@param _cstUseTLFddt - conditional - The study-specific mock table shell
metadata (known as Tables, Listings, and Figures data definition
metadata (TLFDDT)) are used to provide report metadata.
If _cstDisplaySrc=Metadata, either this parameter or
_cstUseAnalysisResults must be set to Y. If both
_cstUseAnalysisResults and _cstUseTLFddt are set to Y,
_cstUseAnalysisResults takes precedence.
Values: N | Y
Default: Y
@param _cstDisplayID - conditional - The ID of the display from the designated
metadata source. If _cstDisplaySrc=Metadata, this parameter is
required.
@param _cstDisplayPath - optional - A valid filename or the fully qualified
path to the generated display. If not provided, the code looks in
SASReferences for type=report.The SAS Clinical Standards
Toolkit ADaM reporting methodology uses a report.properties file to
specify the default report format. By default, the property (and global
macro variable) _cstDefaultReportFormat is set to PDF. Submitting
the analyze_data.sas driver program produces the specified statistical
displays and generates a process results data set. Here is a sample
results data set:
Sample Results Data Set Generated by the analyze_data.sas Driver
Program
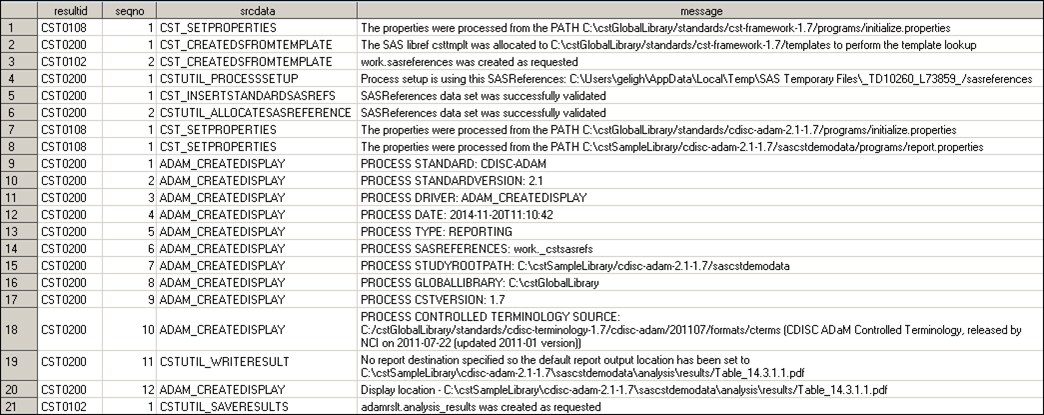
Analysis Results (Tables, Listings, and Figures)
Each generated statistical
display should correspond to a table shell, as described in the TLF
Metadata section. (See SAS Clinical Standards Toolkit Sample Table Shell.)
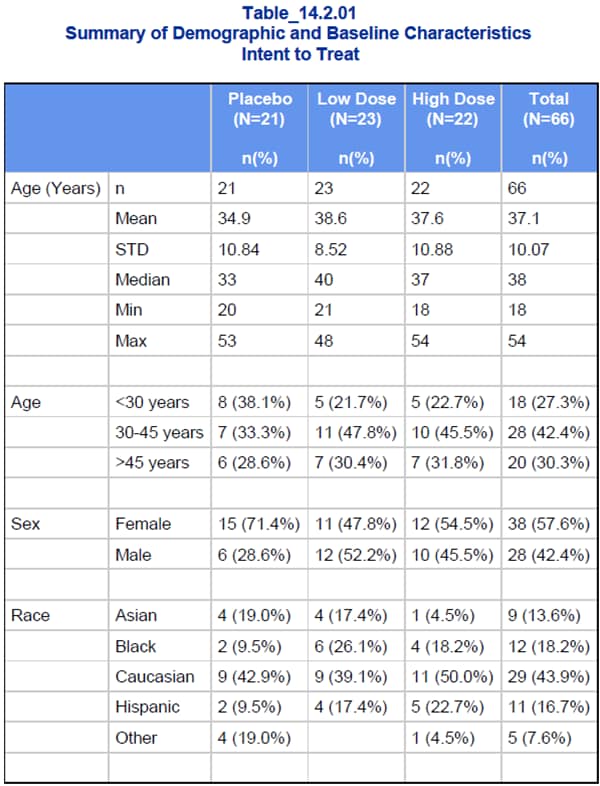
For example, the Summary
of Demographic and Baseline Characteristics provided in Table 14.2.01
is shown in this figure.
Sample Analysis Report: Table 14.2.01
Analysis Results Metadata
The Analysis
Data Model (ADaM), Version 2.1 document provides specifications
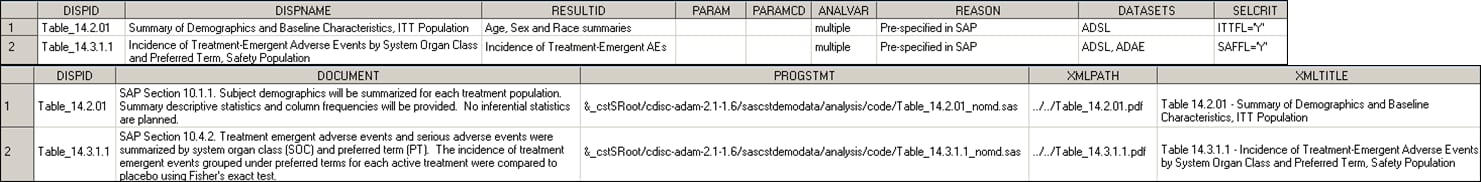
for capturing analysis results. As a result, traceability back to
the contributing source data is possible. Analysis Results Metadata identifies the columns to be included in the analysis results
data set. All analysis results metadata for the
two statistical displays provided with the SAS Clinical Standards
Toolkit is shown in this figure:
Analysis Results Metadata
The analysis results
data set is located here:
sample study library directory/cdisc-adam-2.1–1.7/sascstdemodata/metadata/analysis_results.sas7bdatCopyright © SAS Institute Inc. All Rights Reserved.