SAS Representation of Standards
Overview
The SAS Clinical Standards
Toolkit is designed to support various clinical standards. The SAS
Clinical Standards Toolkit was initially built to support the Clinical
Data Interchange Standards Consortium (CDISC) standards. However,
the generic framework enables definition of any type of standard.
Each SAS Clinical Standards
Toolkit standard provides a SAS representation of the published source
guidelines or source specification. The SAS representation is designed
to serve as a model or template of the source specification.
Two key design requirements
shaped the implementation of the SAS Clinical Standards Toolkit standards.
-
Each supported standard is represented in one or more SAS files. This facilitates these points:
-
It provides SAS users with an implementation of data models and standards that are based on SAS.
-
It enables you to use SAS routines to assess how well any user-defined set of data and metadata conforms to the standard.
-
It enables you to use SAS code to read and derive files in other formats (for example, XML).Each SAS Clinical Standards Toolkit standard is an optimized reference standard from a SAS perspective.
-
-
You are able to define your own customized standards, or you are able to modify existing SAS standards. For more information about how new standards are registered in the SAS Clinical Standards Toolkit, see Registering a New Version of a Standard.
SAS provides new standards
and updates based on customer requirements, changes to source guidelines,
and changes to source specifications.
This document uses the term “reference
standard” to refer to the SAS representation of each source
specification.
The definition of reference
standard depends on several factors, including the complexity of the
external source standard, the intended use of the standard, and your
preferred implementation methodology. Here are three ways to define
reference standard:
-
A limited SAS representation of an external standard, defined as one or more SAS files.For example, consider two of the CDISC standards supported in the SAS Clinical Standards Toolkit. Each CDISC Controlled Terminology standard can be represented in its simplest form as either a SAS data set or SAS format catalog of acceptable values. Each CDISC SDTM standard can be represented as a set of domains (SAS data sets), and as an associated set of data sets that describe the data set and column metadata for those domains. For some users, this might be the only information about the standards needed from the SAS Clinical Standards Toolkit.
-
A distinct folder hierarchy within the global standards library, comprising the previous definition and any supporting files required by the SAS Clinical Standards Toolkit.By default, reference standards are specified in the global standards library that is created when the SAS Clinical Standards Toolkit is deployed. Each reference standard can be unique in regard to the folder hierarchy and supporting files. Consider the CDISC SDTM standard.The following display shows the global standards library folder hierarchy that is provided for CDISC SDTM:Global Standards Library Folder Hierarchy
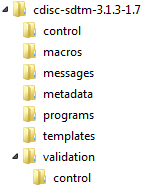 The
Themetadatafolder contains the data set and column metadata for each supported domain. The SAS Clinical Standards Toolkit provides a utility macro (%CST_CREATETABLESFORDATASTANDARD) that reads this metadata, and builds an empty data set for each supported SDTM domain. All supporting files required by the SAS Clinical Standards Toolkit to support the specific CDISC SDTM standard are provided in the remaining folders.-
The
controlfolder provides these data sets:Standards is a single-record file that provides metadata about the standard. Standardlookup provides acceptable values for many discrete-value columns for a number of standard metadata files. StandardSASReferences is a sample or template specification of records that describes input or output files relevant to using the standard. -
The
macrosfolder contains any SAS code specific to the CDISC SDTM standard. -
The
messagesfolder contains messages that are associated with tasks (such as validation) that are supported by the SAS Clinical Standards Toolkit. -
The
metadatafolder provides these data sets:class_tables identifies a limited set of column collections specific to one or more SDTM domains. class_columns identifies the full set of column definitions used in the SDTM domains. reference_tables provides metadata for the specific data sets (domains) that are supported for CDISC SDTM. This information is different for each version of the CDISC SDTM standard. reference_columns provides metadata for the specific columns in the domains that are supported for CDISC SDTM. This information is different for each version of the CDISC SDTM standard. -
The
programsfolder contains several properties files that specify generic SAS Clinical Standards Toolkit properties and specific CDISC SDTM properties translated into SAS global macro variables for a SAS Clinical Standards Toolkit process. -
The
validation/controlfolder provides check metadata that is associated with the primary CDISC SDTM task supported by the SAS Clinical Standards Toolkit.
Each of these folders is discussed in greater detail in this document. -
-
A logical set of files from multiple SAS libraries and multiple standards as defined in the previous two definitions. These are all collated within a single SASReferences data set.Each reference standard can be defined by the files itemized in a SASReferences data set and used to perform a standard task. The SASReferences data set documents all of the input and output files that are associated with a SAS Clinical Standards Toolkit process. These files do not need to be limited to a single standard or be resident in a single standard folder hierarchy. Consider a SASReferences data set that supports a process that builds a CDISC CRT-DDS define.xml file. That SASReferences data set might point to CDISC SDTM source data and metadata, a CDISC controlled terminology SAS format catalog, a set of reference table and column metadata documenting the SAS data sets used to build the define.xml file, and a default style sheet for the generated define.xml file. A broader view of what comprises the CDISC CRT-DDS reference standard must recognize that the standard also references data and metadata from other standards.
Tip
Best Practice Recommendation:
Instead of changing an existing SAS standard, you should define a
new standard. This allows seamless updates to SAS standards, which
facilitates operational qualification, demo scripts, and Technical
Support debugging a fixed standard. There is a way for you to request
a change to an existing standard if there are errors. To
define a new standard, which can be just changing an existing standard
and saving it as a new standard, see Framework.Copyright © SAS Institute Inc. All Rights Reserved.