Special Topic: Using Alternative Controlled Terminologies
The SAS Clinical Standards
Toolkit supports using any set of controlled terminology or any coding
dictionaries such as MedDRA or WHO Drug.
Generally, controlled
terminology is defined to the SAS Clinical Standards Toolkit as SAS
format catalogs, and coding dictionaries as SAS data sets, although
either format is allowed. A SASReferences data set documents all of
these, and facilitates run-time references to the input sources. In
the SAS Clinical Standards Toolkit sample drivers, a SASReferences
type=fmtsearch record points to each SAS format catalog (and allows
specification of a reference order for like-named formats). And, a
type=referencecterm record points to each specific coding dictionary
to be referenced. The format search path is set with a call to the
cstutil_processsetup utility macro.
Consider the following
scenarios and how each one can be handled using the SAS Clinical Standards
Toolkit:
-
Scenario 1: You want to create and manage codelists (SAS formats) independent of the CDISC-Terminology standard provided with SAS Clinical Standards Toolkit.This scenario assumes you have one or more user-defined SAS format catalogs that contain valid values associated with your data columns. These user-defined format catalogs might include extensions to existing CDISC-Terminology codelists or to new formats associated with columns in custom domains. The SAS Clinical Standards Toolkit SASReferences data set enables you to specify references to multiple catalogs and to manage the order in which these appear in the format search path. For example, if you have a catalog named MYTERMS that contains all formats of interest for your study, your SASReferences data set can contain a single type=fmtsearch record:However, if you prefer to keep your customizations in a separate format catalog, but you want to use the CDISC-Terminology codelists provided by SAS, your SASReferences data set will have multiple type=fmtsearch records, with the order column value set to establish the format search path precedence:
-
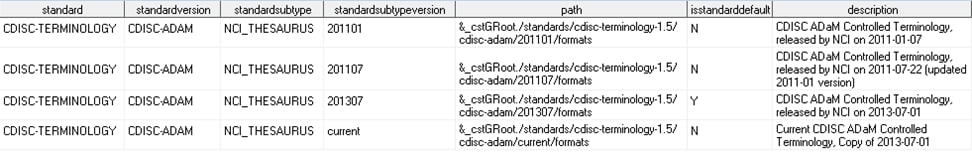
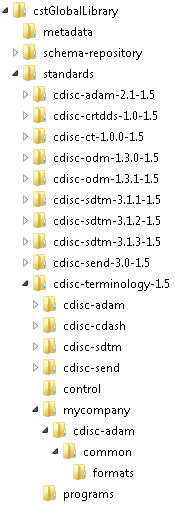
Scenario 2: You want to manage codelist (SAS format) customizations as a registered standard in the global standards library of the SAS Clinical Standards Toolkit.SAS provides snapshots of the CDISC Terminology standard, as provided by the National Cancer Institute (NCI) Enterprise Vocabulary Services (EVS). These snapshots are defined in the global standards library. In the SAS Clinical Standards Toolkit 1.5, these are provided (by CDISC model and snapshot date) in the following location:Consider whether you want to add a new version (such as a dated snapshot) or a completely new set of terminology to the global standards library. To add a new version, follow the snapshot folder hierarchy in the global standards library, and register your new standard in the standardsubstypes data set located in the
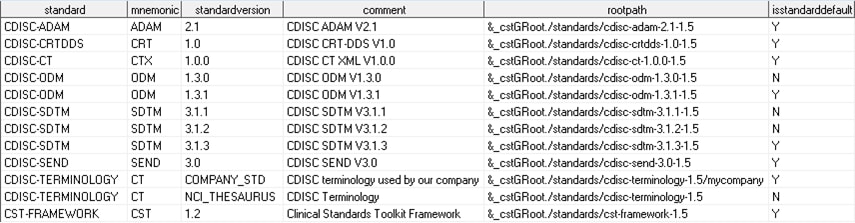
global standards library directory/standards/cdisc-terminology-1.5/controlfolder.For example, suppose you want to add a new CDISC ADaM controlled terminology snapshot released on 01July2013. A new 201307 folder hierarchy is created in the global standards library, a new record is added to the standardsubstypes data set, and the format catalog in the Current subfolder is replaced with the 201307 catalog.The SAS Clinical Standards Toolkit 1.5 provides sample programs that create the data sets that are needed to register controlled terminology. The programs also register these data sets. The programs are called create_terminology_standarddatasets.sas and registerstandard.sas and are located in theglobal standards library directory/standards/cdisc-terminology-1.5/programsfolder.If you want to add a completely new set of terminology to the global standards library, you must follow the information in Maintenance Usage Scenarios.Assume that your organization has created its own comprehensive set of CDISC controlled terminology, and you have created the following global standards library subfolder hierarchy (with CDISC ADaM fully expanded):After the registration process, your global standards library data set might look like this (using the folder hierarchy above): -
Scenario 3: You use multiple versions of the MedDRA dictionary to code Adverse Events across multiple studies within a submission.The SAS Clinical Standards Toolkit does not provide copies of the MedDRA coding dictionary as maintained and distributed by the Maintenance and Support Services Organization. Your organization more than likely maintains the multiple updates to MedDRA, and you might need to reference multiple versions of MedDRA in a single SAS Clinical Standards Toolkit process.Although it is possible to create and use SAS format catalogs for MedDRA lookups (and similar coding dictionary lookups), the SAS Clinical Standards Toolkit provides a mechanism to reference and use a data set lookup methodology in the SASReferences data set using one or more type=referencecterm records. Each record points to a specific MedDRA version using a unique SAS libref, with the resulting libref.dataset available for use, as needed.
-
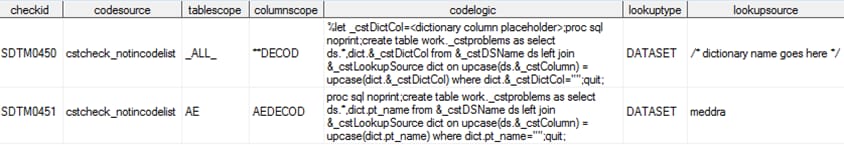
Scenario 4: You use the WHO Drug dictionary to ensure that your coding of Concomitant Medications in CMDECOD and CMCLASCD includes valid terms and class codes.The SAS Clinical Standards Toolkit does not provide copies of the WHO Drug dictionary as created by the World Health Organization and managed by the Uppsala Monitoring Centre. As in Scenario 3, the SAS Clinical Standards Toolkit provides a mechanism to reference and use a data set lookup methodology in the SASReferences data set using one or more type=referencecterm records. Your WHO Drug reference might look like this:The SAS Clinical Standards Toolkit provides several CDISC SDTM validation checks that involve lookups to coding dictionaries. Relevant metadata columns from the validation check data set are listed:The codelogic value is specific to the coding dictionary. In a WHO Drug lookup, drugname and atc_code (or their equivalents) are used. The cstcheck_notincodelist check macro retrieves and uses the lookup data set named in the lookupsource metadata column based on information stored in the SASReferences data set records where type=referencecterm.
Copyright © SAS Institute Inc. All rights reserved.