Special Topic: Identifying Unsupported Elements and Attributes in a CDISC ODM File
Overview
Note: The process explained below
is the same for all ODM versions that are supported by the SAS Clinical
Standards Toolkit. The process is explained using ODM version 1.3.0.
In practice, vendor
and custom extensions to ODM are common. For example, Electronic Data
Capture (EDC) vendors use data management features and flags that
might be exported using ODM XML extensions. By default, such extensions
are ignored by the SAS Clinical Standards Toolkit. Recall that the
SAS Clinical Standards Toolkit uses XSL style sheets for each of the
default, supported 66 ODM data sets (such as ItemDefs.xsl). These
style sheets look for specifically named tags and hierarchical paths
based on the CDISC ODM 1.3.0 published specification. If elements
or attributes exist in the XML file but not in the specification,
they are ignored.
For example, in this
XML code fragment, note the Vendor:<name> syntax.
This represents a hypothetical extension to the ODM XML, presumably
accompanied by a namespace reference supporting the Vendor naming
convention.
<FormData FormOID=" FormDefs.OID.Death" FormRepeatKey="00-01"
TransactionType="Remove" Vendor:Revised="No">
<Vendor:DataQuery DQOID="DQ.OID.001"
QueryText="Premature report of patients demise?">
<Flag>Y</Flag>
<AuditRecord>
<UserRef UserOID="User.OID.I024" />
<LocationRef LocationOID="Location.OID.S001" />
<DateTimeStamp>2011-01-24T15:13:22</DateTimeStamp>
</AuditRecord>
</Vendor:DataQuery>
</FormData>In this code fragment,
the Vendor:DataQuery syntax specifies a new element with several new
attributes and references to other existing (supported) elements.
Note also the additional Vendor:Revised attribute for FormData.
The SAS Clinical Standards
Toolkit provides a utility macro to parse the ODM XML file to identify
currently unsupported elements and tags. This macro, cstutil_readxmltags,
is located in the primary SAS Clinical Standards Toolkit autocall
library (
!sasroot/cstframework/sasmacro).
%cstutil_readxmltags(
_cstxmlfilename=inxml
,_cstxmlreporting=Dataset
,_cstxmlelementds=work.cstodmelements
,_cstxmlattrds=work.cstodmattributes);In this call, the XML
file to be parsed is specified with the inxml fileref. The results
of the parsing are to be written to two data sets, work.cstodmelements
for all unique elements found in the XML file and work.cstodmattributes
for all unique attributes found associated each unique element.
Sample Utility Program: find_unsupported_tags.sas
Overview
The SAS Clinical Standards
Toolkit provides a utility program, find_unsupported_tags.sas, to
demonstrate assessment of the ODM XML file elements and attributes.
This program is located in:
This program provides
the same process setup function supported in most SAS Clinical Standards
Toolkit driver modules, building a SASReferences data set that defines
process inputs and outputs, and allocating all SAS librefs and filerefs.
Here is the general
workflow of this utility program:
-
Build a process-specific SASReferences data set.
-
Call the %cstutil_processsetup() macro to set process paths and perform required library and file allocations.
-
Call the cstutil_readxmltags macro to create a data set of element names and a data set of attribute names.
-
Compare elements and attributes to a set of known (for example, supported) elements and attributes.
-
Report discrepancies.
The SASReferences Data Set
As a part of
each SAS Clinical Standards Toolkit process setup, a valid SASReferences
data set is required. It references the input files that are needed,
the librefs and filenames to use, and the names and locations of data
sets to be created by the process. It can be modified to point to
study-specific files. For
an explanation of the SASReferences data set, see SASReferences File.
In the SASReferences
data set, three input references and one output reference are key
to successful completion of the find_unsupported_tags.sas utility
program. Key Components of the SASReferences Data Set for the find_unsupported_tags.sas Macro lists these
files and data sets, and they are discussed in separate sections.
In the sample find_unsupported_tags.sas
utility program, these values are set for &studyRootPath and &studyOutputPath:
Process Inputs
The metadata type externalxml
refers to the ODM XML file that is being read. The filename odmxml
is defined in the SASReferences data set. This filename is used in
the submitted SAS code when referring to the XML file. The ODM XML
file odm_extended.xml contains sample extensions to the core ODM 1.3.0
model.
The metadata type standardmetadata,
referenced by the odmmeta SAS libref, references the
global standards library directory/standards/cdisc-odm-1.3.0-1.5/metadata folder.
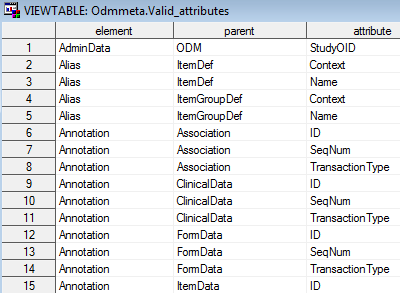
This folder includes the two data sets valid_elements and valid_attributes,
which contain the full list of ODM core elements and attributes supported
by the SAS Clinical Standards Toolkit. The valid_elements data set
contains a single column element itemizing the ODM core elements.
The valid_attributes data set contains each attribute within the context
of its parent tag and containing element.
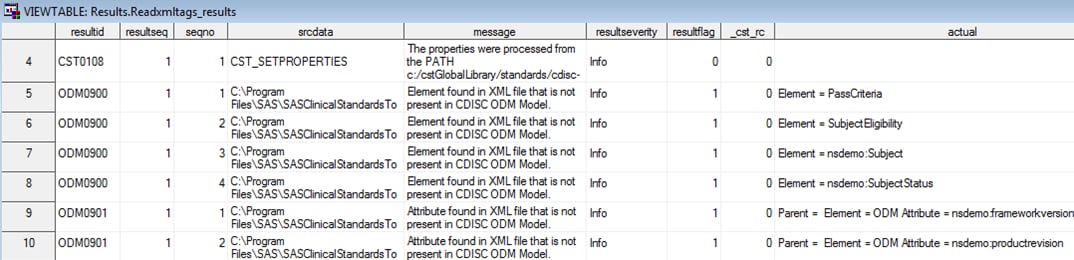
Process Outputs
The results type refers
to the Results data set that contains information from running the
process. In the SAS Clinical Standards Toolkit sample code hierarchy,
this information is written to the
sample study library directory/cdisc-odm-1.3.0–1.5/results directory.
This location is represented in the utility program by the Results
library name.
Depending on the parameter
values associated with the call to the cstutil_readxmltags macro,
two additional process outputs might be persisted at the conclusion
of the process. If the _cstxmlreporting parameter is set to Dataset,
any unsupported elements are documented in the data set referenced
by the _cstxmlelementds parameter and any unsupported attributes are
documented in the data set referenced by the _cstxmlattrds parameter.
Copyright © SAS Institute Inc. All rights reserved.