Building a SASReferences File
Each SASReferences file
requires content that is specific to its planned use. For example,
a SAS Clinical Standards Toolkit process that creates a define.xml
file requires the specification of XML and recommends the specification
of style sheet information. A SAS Clinical Standards Toolkit process
that validates data against a standard requires the specification
of the validation checks to be run.
The SAS Clinical Standards
Toolkit offers several ways to create a SASReferences file for use
in subsequent processes.
-
Use sample SASReferences files that are provided with the SAS Clinical Standards Toolkit. These sample SASReferences files contain the required and optional contents for specific tasks. For example, the task of validating the functionality of CDISC SDTM 3.1.2 uses the SASReferences file found in this location in SAS 9.3:An excerpt of this sample SASReferences file is provided in A Sample SASReferences Data Set.
-
The SAS Clinical Standards Toolkit provides SASReferences templates for use. These templates are either zero-observation data sets or data sets containing records that must be modified. A SASReferences data set template is here:The SAS Clinical Standards Toolkit provides default SASReferences data sets for each supported standard. These default SASReferences data sets contain records that are commonly required for certain SAS Clinical Standards Toolkit tasks (such as validation). However, all records that are required might not be included. Or, all records that are included might not be required for certain tasks. And, SAS librefs, filerefs, paths, and memname values might require modification. For example, see the StandardSASReferences data set found in:
-
The SAS Clinical Standards Toolkit provides the utility macros to build and return many SAS Clinical Standards Toolkit metadata data sets.
-
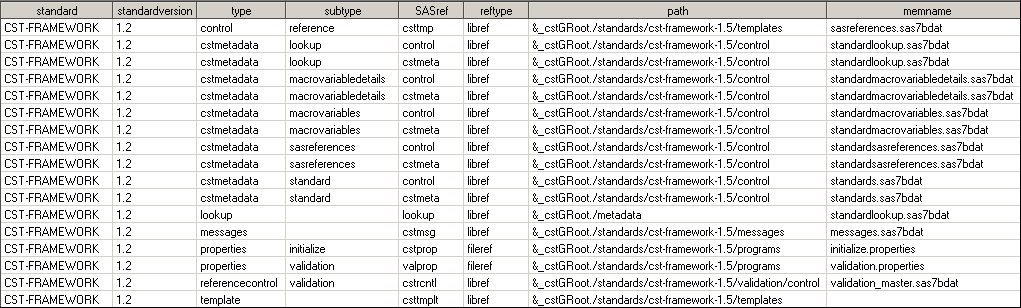
The %cst_getstandardsasreferences macro returns the StandardSASReferences data set. (See the file description in Metadata File Descriptions for the specified standard.)
-
The
primary function of the SASReferences file is to define the SAS Clinical
Standards Toolkit process inputs and outputs. What information does
the process need to reference? What does the process produce? Where
does the information come from and go? The “what” information
is determined by the use of two SASReferences fields: type and subtype.
The “where” information is determined by path and memname.
The values for all of these fields are restricted for the SAS Clinical
Standards Toolkit to values itemized in the framework Standardlookup
data set found in:
Customizing the type
and subtype values in the Standardlookup data set is allowed. Customization
is a prerequisite if you want to use the field values in any SASReferences
data set that is used by the SAS Clinical Standards Toolkit.
This table lists and describes
the acceptable type and subtype values in the framework Standardlookup
data set.
Every instance of the
SASReferences file does not require a specific path and filename.
At the beginning of this section, a call to this macro was described:
%cst_getstandardsasreferences(_cstStandard=CST-FRAMEWORK, _cstStandardVersion=1.2,_cstOutputDS=sasreferences);
The standard SASReferences
data set has been expanded in the SAS Clinical Standards Toolkit 1.5.
The SASref field, with values of cstmeta and control,
points to the same path field value. The SAS Clinical Standards Toolkit
1.5 was enhanced with new features, and the control SASref
was retained to ensure backward compatibility with past releases.
In addition, there are
new memnames, such as standardmacrovariabledetails.sas7bdat and standardmacrovariables.sas7bdat.
These are new data sets that contain metadata about the SAS Clinical
Standards Toolkit 1.5 global macro variables. They are used for internal
validation.
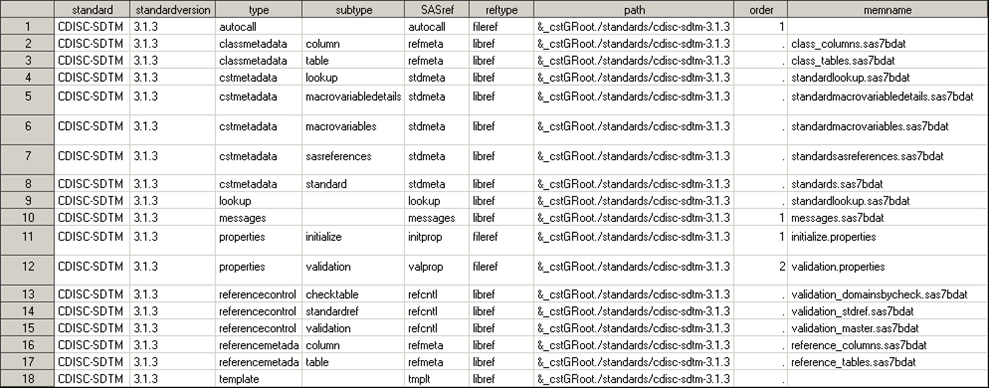
Standard SASReferences for CDISC SDTM shows the information returned by
this call to %cst_getstandardsasreferences for the CDISC SDTM standard:
A comparison
of Standard SASReferences File for CST-FRAMEWORK and Standard SASReferences for CDISC SDTM shows little similarity in the record types and no overlap
in references to specific files. The target inputs and outputs for
CDISC SDTM are more focused on the task (for example, validating SDTM
domains). The SAS Clinical Standards Toolkit validation processes
require specification of a comparative reference standard. Here, there
are references to a standard-specific macro library (autocall), Messages
data set, and properties files. Unique SASref values by type are provided,
pointing to distinct files and folders in the global standards library.
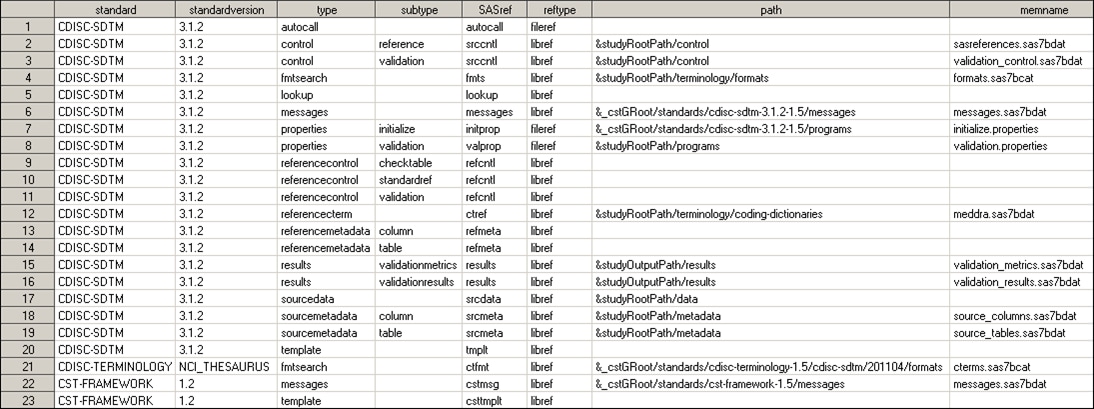
Consider an actual SASReferences
file built to support CDISC SDTM 3.1.2 validation. The task of validating
the functionality of CDISC SDTM 3.1.2 uses the SASReferences file
in this location in SAS 9.3:
|
Illustrates the call
to a standard-specific properties file that is used to initialize
a global macro variable that is specific to that standard. Referencing
a standard-specific properties files in the SASReferences data set
is recommended. The call to the CST-FRAMEWORK initialize.properties
file is a prerequisite setup step outside of SASReferences and performed
before processing SASReferences.
|
|
|
Points to
the reference standard for CDISC SDTM 3.1.2, but unlike the template
defaults in Standard SASReferences for CDISC SDTM, path and memname are blank. Leaving
them blank tells the SAS Clinical Standards Toolkit to look in the
CDISC SDTM 3.1.2 StandardSASReferences file and use the defaults for
that standard and version. This convention facilitates portability
of the data set by doing a run-time lookup for the current information. The lookup
results in the inclusion of the path and memname values as defined
in Standard SASReferences for CDISC SDTM.
|
|
|
This is a new type not
in the template files (StandardSASReferences). It defines the location
of the study (source) data. The use of &studyRootPath, coupled
with the assumption of a fixed-folder hierarchy, enables portability
across studies. The memname value is not relevant for a library of
SAS data sets.
|
|
An alternative way to
build the SASReferences file is to use the %cst_createdsfromtemplate
utility macro.
%cst_createdsfromtemplate(_cstStandard=CST-FRAMEWORK,_cstType=control, _cstSubType=reference,_cstOutputDS=work.sasreferences); proc sql; insert into work.sasreferences values(CST-FRAMEWORK 1.2 messages messages libref 1 ); . . . quit;
This macro copies the
template. New records can be added various ways, including the previous
PROC SQL technique. There is no requirement that the SASReferences
file has to live outside the SAS Work area and be kept beyond the
SAS Clinical Standards Toolkit process. However, these are best practices
that enable future capabilities such as process reruns and reporting.
Copyright © SAS Institute Inc. All rights reserved.