Global Standards Library
The global standards
library is the metadata repository for the SAS Clinical Standards
Toolkit. By default, the global standards library contains the metadata
for the Framework module and the metadata for each data standard that
is provided by SAS (such as the CDISC SDTM 3.1.2 standard).
During the installation and configuration
of the SAS Clinical Standards Toolkit, you are prompted for the location
where the global standards library should be installed. The configuration
process creates a series of directories in this location.
-
metadatacontains data sets that have information about the registered standards. For more information, see Common Framework Metadata.
The
metadata directory contains two data sets: Standards
and StandardSASReferences. The Standards data set has a list of the
registered standards and basic information relating to each standard.
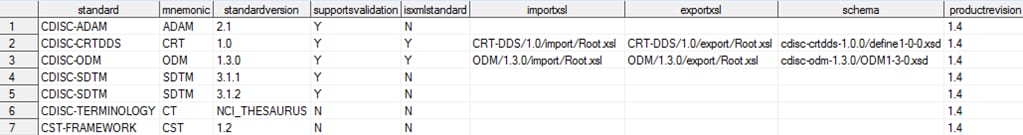
This display provides
the full content of the global standards library Standards data set
included with the SAS Clinical Standards Toolkit after a new installation
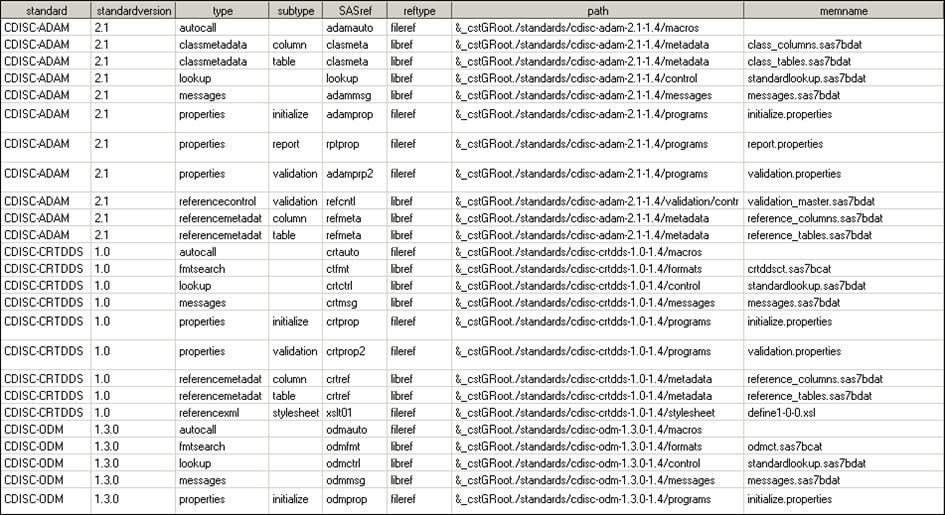
of the application. (The columns are continued in the second image.)
Global Standards Library: Metadata Standards Data Set
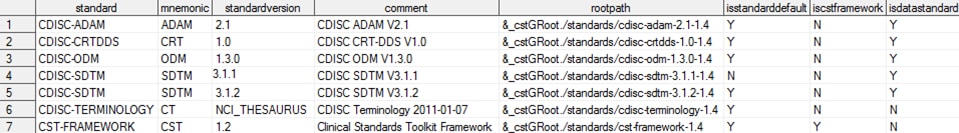
Note: The
&_cstGRoot directory in the rootpath column maps to
the <global standards library directory>.
The StandardSASReferences
data set defines the typical inputs and outputs of SAS processes that
are associated with each standard.
The type and subtype columns can be used to reference
information that the SAS Clinical Standards Toolkit needs. This information
is in the directory structures and file naming standards used by the
customer. A full list of valid types and subtypes are provided in
this document.
The
standards directory contains subdirectories for each
of the standard versions that is provided by SAS. In addition, there
are subdirectories for user-customized versions of these standards
and any new user-defined standards. Each subdirectory should be considered
a stand-alone module. This is how the SAS Clinical Standards Toolkit
can keep parallel standards and reduce the need for revalidation.
Within each subdirectory, there might be directories that group the
files, data sets, and housekeeping programs.
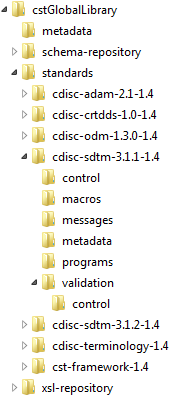
This display shows
the directory structure for a Microsoft Windows global standards library
with cdisc-sdtm-3.1.1-1.4 expanded.
Directory Structure for a Microsoft Windows Global Standards
Library
The
schema-repository directory contains
XML schema definitions that are used to validate XML files. Standards
that use XML should have their schemas in this directory so that they
can be found. For example, the schema-repository directory for CDISC CRT-DDS 1.0 as defined in the Standards data
set maps to: