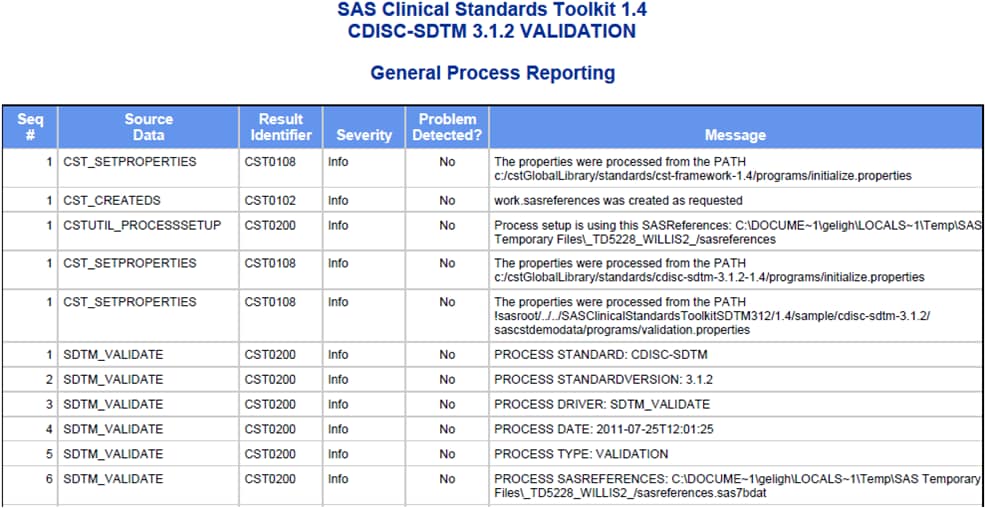
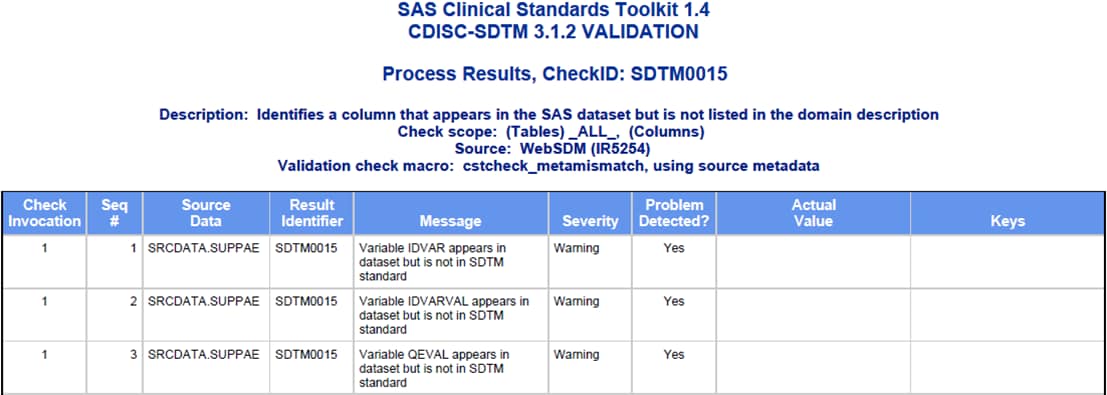
Process Results Reporting
Reports 1 and 2 have
multiple sections or panels. Each section can be optionally generated.
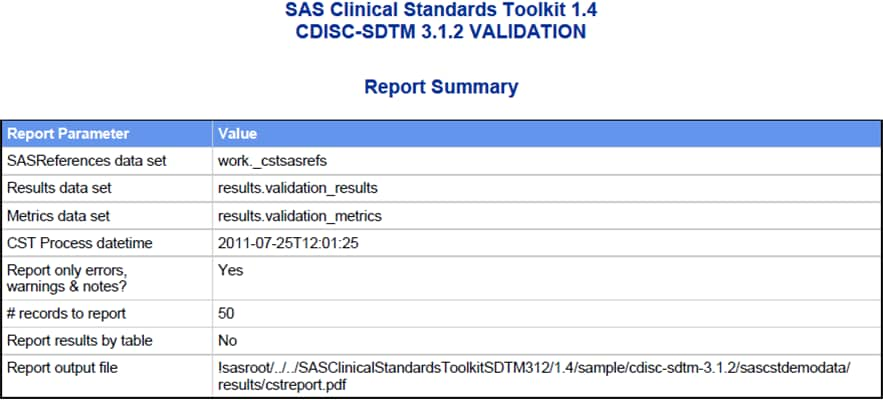
Several sections are common to each report, including a report summary,
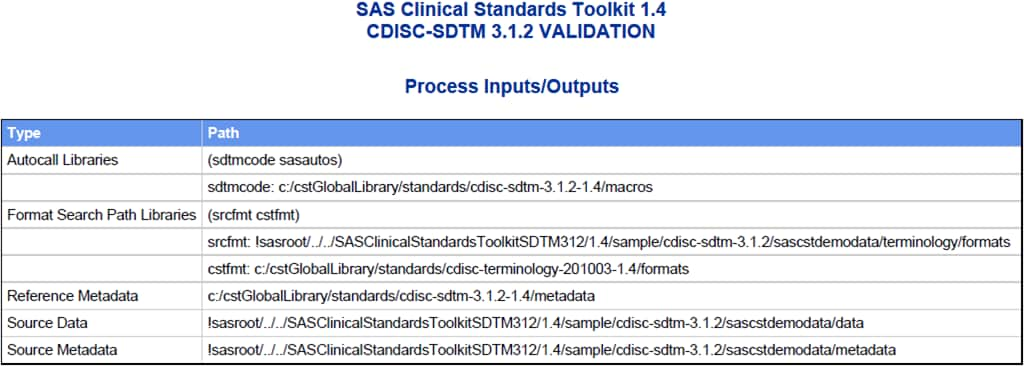
a listing of key process inputs and outputs as defined in the SASReferences
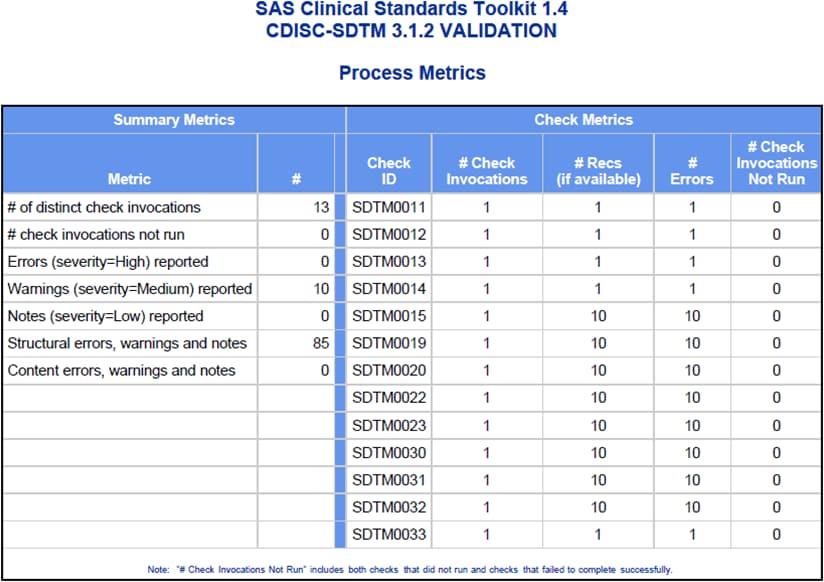
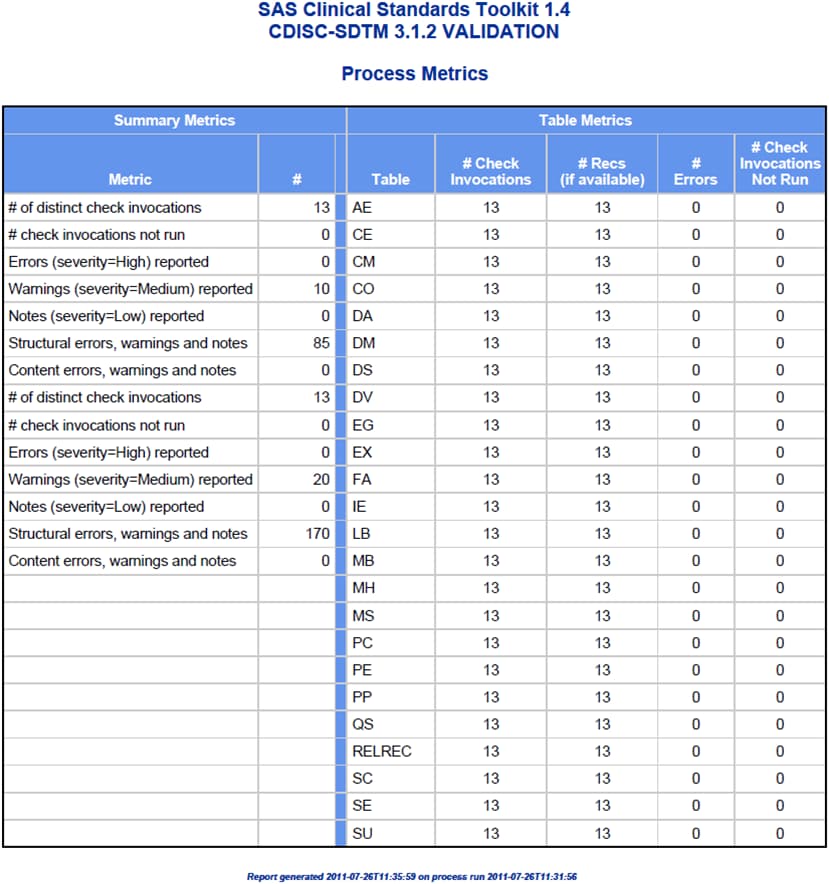
data set, a summary of validation metrics, and a general process messaging
panel.
A sample driver program
is provided to define the SAS Clinical Standards Toolkit environment
and to call the primary task framework macro (%cstutil_createreport).
This excerpt from the driver program header provides a brief overview:
cst_report.sas
Sample driver program to perform a primary Toolkit action, in this case,
reporting of process results. This code performs any needed set-up and data
management tasks, followed by one or more calls to the %cstutil_createreport()
macro to generate report output.
Two options for invoking this routine are addressed in these scenarios:
(1) This code is run as a natural continuation of a CST process, within
the same SAS session, with all required files available. The working
assumption is that the SASReferences data set (referenced by the
_cstSASRefs macro) exists and contains information on all input files
required for reporting.
(2) This code is being run in another SAS session with no CST setup
established, but the user has a CST results data set and therefore can
derive the location of the SASReferences file that can provide the full
CST setup needed to run the reports.
Assumptions:
To generate all panels for both types of reports, the following metadata
is expected:
- the SASReferences file must exist, and must be identified in the
call to cstutil_processsetup if it is not work.sasreferences.
- a Results data set.
- a (validation-specific) Metrics data set.
- the (validation-specific) run-time Control data set itemizing the
validation checks requested.
- access to the (validation-specific) check messages data set.The reporting as implemented
in the SAS Clinical Standards Toolkit attempts to address these two
scenarios described in the driver module header above:
-
Some SAS Clinical Standards Toolkit task (such as validation against a reference standard) has been completed. The Results data set has been created. And, in the same SAS session (or batch job stream), you want to generate one or both reports. In this scenario, the reporting process uses the SASReferences data set defined by the global macro variable _cstSASRefs that was used by the previous process. The Results data set to be summarized in the report is the data set that was previously created and perhaps persisted to a location other than the SAS Work library. (Whether the data set was persisted was specified in the SASReferences data set.) Other files required by the report are identified in Metadata Sources for Reporting.TipBest Practice Recommendation: The cleanup macro, %cstutil_cleanupcstsession, should not be called between primary tasks in a SAS Clinical Standards Toolkit SAS session (such as between validation and reporting). This keeps required files, macro variables, autocall paths, and so on, available for the reporting code.
-
The Results data set that was created in some prior SAS Clinical Standards Toolkit session is available. You want to generate one or both reports. The SAS Clinical Standards Toolkit processes add informational records to the Results data set, documenting the process itself. For example, a SAS Clinical Standards Toolkit CDISC SDTM validation process writes records to the Results data set that contains this sample message text:
Message PROCESS STANDARD: CDISC-SDTM PROCESS STANDARDVERSION: 3.1.1 PROCESS DRIVER: SDTM_VALIDATE PROCESS DATE: 2010-01-25T11:56:17 PROCESS TYPE: VALIDATION PROCESS SASREFERENCES: !sasroot/../SASClinicalStandardsToolkitSDTM311/ 9.1.3/sample/cdisc-sdtm- 3.1.1/SASDemo/control/sasreferences.sas7bdatFrom this information, a reporting process can attempt to find and open the referenced SASReferences data set to derive information for some or all of the report sections.CAUTION:There are obvious limits to how useful any SAS Clinical Standards Toolkit Results data set can be in rebuilding a session for reporting purposes.For example, if the SASReferences data set was built in the Work library in a previous session, then it will not be available and the report process fails. Similarly, if the SASReferences data set references library and file paths using a macro variable prefix (for example, &_cstGRoot or &studyRootPath), and those macro variables are not set or point to a different root path than the original process, then the report process might fail or yield unpredictable results. In the example above, the referenced SASReferences data set points to a!sasroot folder hierarchy that was used for a SAS Clinical Standards Toolkit 1.2 process. This folder hierarchy no longer exists in the SAS Clinical Standards Toolkit 1.4, so the results data set would not be found. This scenario or technique is most appropriate for sites that adopt a consistent means of building and populating SASReferences data sets.
Note: Beginning in the SAS Clinical
Standards Toolkit 1.3, you are able to define report output locations
in the SASReferences data set. These locations can be defined with
type=report in SASReferences. They can be further specified in the
framework Standardlookup data set. For more information,
see Framework.
This code is excerpted
from the cst_report.sas driver module and performs the setup tasks
that are specific to reporting:
* Initialize macro variables used for this task *; %let _cstRptControl=; %let _cstRptLib=; %let _cstRptMetricsDS=; %let _cstRptOutputFile=&studyOutputPath/results/cstreport.pdf; %let _cstRptResultsDS=; %let _cstSetupSrc=SASREFERENCES; %let _cstStandard=CDISC-SDTM; %let _cstStandardVersion=3.1.2; %cstutil_processsetup(_cstSASReferencesLocation=&studyrootpath/control); %cstutil_reportsetup(_cstRptType=Results);
An alternative
setup to support Scenario 2, as described, would include these code excerpts:
%let _cstSetupSrc=RESULTS; %cstutil_processsetup(); %let _cstRptResultsDS=work.validation_results; %cstutil_reportsetup(_cstRptType=Results);
As the final step, the
reporting driver program makes one or more calls to the utility reporting
macro. At a minimum (using default parameter values), a simple macro
call to create report 2 might include this code:
%cstutil_createreport(_cstsasreferencesdset=&_cstSASRefs,_cstreportbydomain=Y, _cstreportoutput=&studyrootpath/results/cstchecktablereport.pdf);
Supported Parameters for the %cstutil_createreport Macro
|
If null (default), then
this parameter reports all records in error (where results.resultflag=1)
in the Results data set. Otherwise, set this parameter to any integer
value > 0, signifying the number of records to print per checkid
(where results.checkid is non-null). If _cstreportobs > 0 excludes
any records, then a footnote is printed, noting that not all records
were printed.
|
|
|
Report 2 parameter.
A data set that provides a list of tables for each check. Using this
parameter assumes that this data set has been built before running
this report. For more information,
seeSupplemental Validation Check Metadata: Domains by Check. This parameter is optional. If this parameter
is not used, then the data set is created.
|
|
A more complete example
of the %cstutil_createreport reporting macro includes this macro call:
%cstutil_createreport( _cstsasreferencesdset=&_cstSASRefs, _cstresultsdset=&_cstRptResultsDS, _cstmetricsdset=&_cstRptMetricsDS, _cstreportbytable=N, _cstreporterrorsonly=Y, _cstreportobs=50, _cstreportoutput=%nrbquote(&_cstRptOutputFile), _cstsummaryReport=Y, _cstioReport=Y, _cstmetricsReport=Y, _cstgeneralResultsReport=Y, _cstcheckIdResultsReport=Y);
Interpretation of this
request, based on the parameter descriptions in Table 9.2, produces
a (validation) results listing that contains all five report panels
and includes only the first 50 errors that are reported for each validation
check.