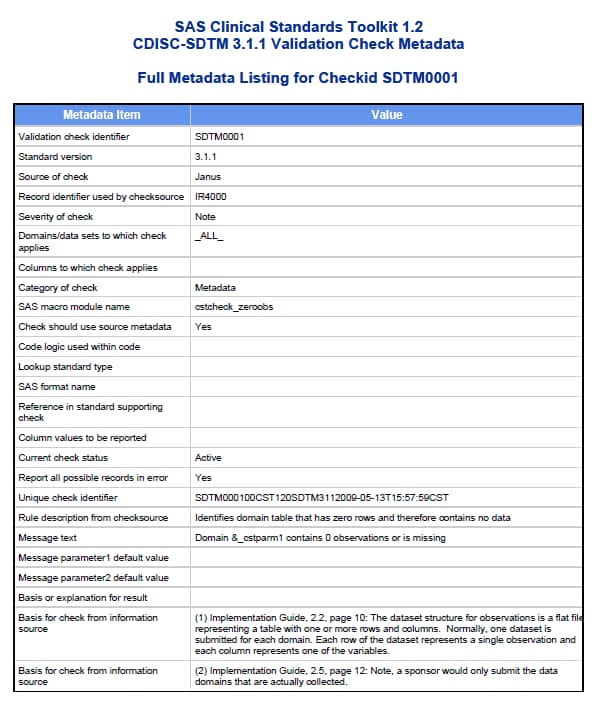
Process Results Reporting
Reports
1 and 2 have multiple sections or panels. Each section can be optionally
generated. Several sections are common to each report, including a
report summary, a listing of key process inputs and outputs as defined
in the SASReferences data set, a summary of validation metrics, and
a general process messaging panel.
A sample
driver program is provided to define the SAS Clinical Standards Toolkit
environment and to call the primary task framework macro (%cstutil_createreport).
The following excerpt from the driver program header provides a brief
overview:
cst_report.sas
Sample driver program to perform a primary Toolkit task, in this case,
reporting process results. This code performs any needed setup and data
management tasks, followed by one or more calls to the %cstutil_createreport()
macro to generate report output.
Two options for invoking this routine are addressed in these scenarios:
(1) This code is run as a natural continuation of a CST process, in
the same SAS session, with all required files available. The working
assumption is that the SASReferences data set (referenced by the
_cstSASRefs macro) exists and contains information on all input files
required for reporting.
(2) This code is run in another SAS session with no CST setup
established, but the user has a CST Results data set and, therefore, can
derive the location of the SASReferences data set that can
provide the full CST setup needed to run the reports.
Assumptions:
To generate all panels for both types of reports, the following metadata
is expected:
- the SASReferences data set must exist and be identified in the
call to cstutil_processsetup if it is not work.sasreferences.
- a Results data set.
- a (validation-specific) Metrics data set.
- the (validation-specific) run-time Control data set itemizing the
validation checks requested.
- access to the (validation-specific) check Messages data set.
The reporting
as implemented in the SAS Clinical Standards Toolkit attempts to address
the following two scenarios described in the driver module header
above:
-
Some SAS Clinical Standards Toolkit task (such as validation against a reference standard) has been completed. The Results data set has been created. And, in the same SAS session (or batch job stream), you want to generate one or both reports. In this scenario, the reporting process uses the SASReferences data set defined by the global macro variable _cstSASRefs that was used by the previous process. The Results data set to be summarized in the report is the data set that was previously created and perhaps persisted to a location other than the SAS Work library. (Whether the data set was persisted was specified in the SASReferences data set.) Other files required by the report are identified in Metadata Sources for Reporting.Best Practice Recommendation: The cleanup macro, %cstutil_cleanupcstsession, should not be called between primary tasks in a SAS Clinical Standards Toolkit SAS session (such as between validation and reporting). This keeps required files, macro variables, autocall paths, and so on, available for the reporting code.
-
The Results data set that was created in some prior SAS Clinical Standards Toolkit session is available. You want to generate one or both reports. The SAS Clinical Standards Toolkit processes add informational records to the Results data set, documenting the process itself. For example, a SAS Clinical Standards Toolkit CDISC SDTM validation process writes records to the Results data set that contains the following sample message text:
Message PROCESS STANDARD: CDISC-SDTM PROCESS STANDARDVERSION: 3.1.1 PROCESS DRIVER: SDTM_VALIDATE PROCESS DATE: 2010-01-25T11:56:17 PROCESS TYPE: VALIDATION PROCESS SASREFERENCES: !sasroot/../SASClinicalStandardsToolkitSDTM311/ 9.1.3/sample/cdisc-sdtm- 3.1.1/SASDemo/control/sasreferences.sas7bdatFrom this information, a reporting process can attempt to find and open the referenced SASReferences data set to derive information for some or all of the report sections.Warning: There are obvious limits to how useful any SAS Clinical Standards Toolkit Results data set can be in rebuilding a session for reporting purposes. For example, if the SASReferences data set was built in the Work library in a previous session, then it will not be available and the report process fails. Similarly, if the SASReferences data set references library and file paths using a macro variable prefix (for example, &_cstGRoot or &studyRootPath), and those macro variables are not set or point to a different root path than the original process, then the report process might fail or yield unpredictable results. This scenario or technique is most appropriate for sites that adopt a consistent means of building and populating SASReferences data sets.
Note: In the SAS
Clinical Standards Toolkit 1.3, you are able to define report output
locations in the SASReferences data set. These locations can be defined
with type=report in SASReferences. They can be further specified in
the framework Standardlookup data set. For more information, see Framework.
The following
code was excerpted from the cst_report.sas driver module and performs
the setup tasks that are specific to reporting:
* Initialize macro variables used for this task *; %let _cstRptControl=; %let _cstRptLib=; %let _cstRptMetricsDS=; %let _cstRptOutputFile=&studyOutputPath/results/cstreport.pdf; %let _cstRptResultsDS=; %let _cstSetupSrc=SASREFERENCES; %let _cstStandard=CDISC-SDTM; %let _cstStandardVersion=3.1.2; %cstutil_processsetup(_cstSASReferencesLocation=&studyrootpath/control); %cstutil_reportsetup(_cstRptType=Results);
An alternative
setup to support Scenario 2, as described on page 196, would include
the following code excerpts:
%let _cstSetupSrc=RESULTS; %cstutil_processsetup(); %let _cstRptResultsDS=work.validation_results; %cstutil_reportsetup(_cstRptType=Results);
As the
final step, the reporting driver program makes one or more calls to
the utility reporting macro. At a minimum (using default parameter
values), a simple macro call to create report 2 might include the
following:
%cstutil_createreport(_cstsasreferencesdset=&_cstSASRefs,_cstreportbydomain=Y, _cstreportoutput=&studyrootpath/results/cstchecktablereport.pdf);
Supported Parameters for the %cstutil_createreport Macro
|
If null (default), then
this parameter reports all records in error (where results.resultflag=1)
in the Results data set. Otherwise, set this parameter to any integer
value > 0, signifying the number of records to print per checkid
(where results.checkid is non-null). If _cstreportobs > 0 excludes
any records, then a footnote is printed, noting that not all records
were printed.
|
|
|
Report 2 parameter.
A data set that provides a list of tables for each check. Using this
parameter assumes that this data set has been built before running
this report. For more information, see Supplemental Validation Check Metadata: Domains by Check. This parameter is optional. If this parameter is not used,
then the data set is created.
|
|
A more
complete example of the %cstutil_createreport reporting macro includes
the following macro call:
%cstutil_createreport( _cstsasreferencesdset=&_cstSASRefs, _cstresultsdset=&_cstRptResultsDS, _cstmetricsdset=&_cstRptMetricsDS, _cstreportbytable=N, _cstreporterrorsonly=Y, _cstreportobs=50, _cstreportoutput=%nrbquote(&_cstRptOutputFile), _cstsummaryReport=Y, _cstioReport=Y, _cstmetricsReport=Y, _cstgeneralResultsReport=Y, _cstcheckIdResultsReport=Y);
Interpretation
of this request, based on the parameter descriptions in Table 9.2,
produces a (validation) results listing that contains all five report
panels and includes only the first 50 errors that are reported for
each validation check.