Writing XML Files
Overview
Support
of CDISC XML-based standards such as CDISC CRT-DDS (define.xml) and
CDISC ODM includes the ability to render these files in SAS data set
format and the ability to create model-specific XML files from a SAS
data set representation of those standards.
In SAS
Clinical Standards Toolkit 1.3, you can create a CDISC CRT-DDS 1.0
define.xml file that references a CDISC SDTM 3.1.1 or 3.1.2 study.
CDISC ODM write capabilities are under development. (For the latest
updates, see the SAS Support Web site for SAS Clinical Standards Toolkit
at
http://support.sas.com/rnd/base/cdisc/cst/index.html).
Creating the CDISC CRT-DDS 1.0 define.xml File
There
are four key macros that are provided with the SAS Clinical Standards
Toolkit that support creation of the define.xml file. The four macros
are listed in the order in which they are executed:
-
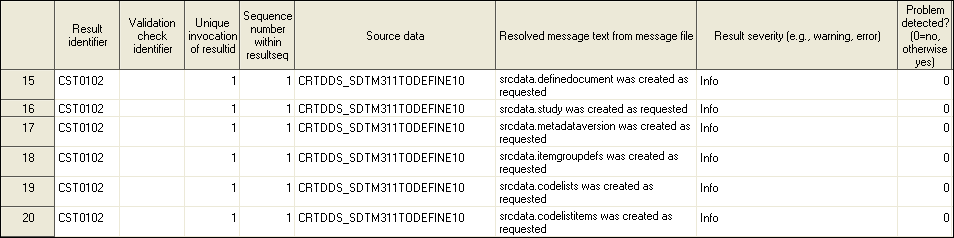
The crtdds_sdtm311todefine10.sas macro creates the 39 tables for the SAS representation of the CRT-DDS files from SDTM metadata. This macro, using SDTM table and column metadata as its source, populates a subset of 12 CRT-DDS data sets. Although the macro name implies that it is specific to SDTM 3.1.1, it operates on both CDISC SDTM 3.1.1 and 3.1.2 domains.
These
macros are called by driver programs that are responsible for properly
setting up each SAS Clinical Standards Toolkit process to perform
a specific SAS Clinical Standards Toolkit task. Three sample driver
modules are provided with the SAS Clinical Standards Toolkit CDISC
CRT-DDS standard. The following lists the purpose of each of these
drivers:
These
three driver programs are examples that are provided with the SAS
Clinical Standards Toolkit. You can use these driver programs or create
your own. The names of these driver programs are not important. However,
the content is important and demonstrates how the various SAS Clinical
Standards Toolkit framework macros are used to generate the required
metadata files.
Sample Driver Program: create_crtdds10_from_sdtm311.sas
Overview
The create_crtdds10_from_sdtm311.sas
driver program sets up the required environment variables and library
references to initiate the crtdds_sdtm311todefine10.sas macro. This
macro extracts data from the SDTM 3.1.1 or 3.1.2 metadata files. (For
more information about the source_tables and source_columns data sets,
see Source Metadata.) Depending on the available source information, the macro
attempts to convert the information into the 39 tables that represent
the SAS interpretation of the CDISC CRT-DDS 1.0 model. All 39 data
sets are created, but only those data sets with the available data
are populated. The other tables contain zero observations.
%crtdds_sdtm311todefine10( _cstOutLib=srcdata, _cstSourceTables=sampdata.source_tables, _cstSourceColumns=sampdata.source_columns, _cstSourceStudy=sampdata.source_study );
In the
example, the crtdds_sdtm311todefine10 macro sets
_cstOutLib to srcdata. All of the CRT-DDS-defined
tables are written to the SAS Srcdata library. The _cstSourceTables parameter accesses the source_tables data set that exists in the
Sampdata library (sampdata.source_tables).
The _cstSourceColumns parameter accesses
the source_columns data set that exists in the Sampdata library (sampdata.source_columns). The _cstSourceStudy parameter accesses the source_study data set that exists in the
sampdata library (sampdata.source_study).
The create_crtdds10_from_sdtm311.sas
driver program is provided with SAS, and it is ready to run on any
of the SDTM sample studies. Although the program name implies that
it is specific to SDTM 3.1.1, it operates on both CDISC SDTM 3.1.1
and 3.1.2 domains. The driver program can be run interactively or
in batch. To run the program interactively, start a SAS session, and
load the driver program into the SAS editor.
!sasroot/../SASClinicalStandardsToolkitCRTDDS10/1.3/sample/cdisc-crtdds-1.0/programs/create_crtdds10_from_sdtm311.sasThe SASReferences Data Set
As a part
of each SAS Clinical Standards Toolkit process setup, a valid SASReferences
data set is required. It can be modified to point to study-specific
files. For an explanation of the SASReferences data set, see SASReferences File.
In the
SASReferences data set, there are two input file references and one
output reference that are key to successful completion of the create_crtdds10_from_sdtm311.sas
driver program. The following table lists these files and data sets,
and they are discussed in separate sections. In the sample create_crtdds10_from_sdtm311.sas
driver program, the following values are set for &studyRootPath
and &studyOutputPath and are specific to a SAS release.
&studyRootPath=!sasroot/../SASClinicalStandardsToolkitSDTM312/1.3/sample/cdisc-sdtm-3.1.2/sascstdemodata&studyRootPath=!sasroot/../../SASClinicalStandardsToolkit SDTM312/1.3/sample/cdisc-sdtm-3.1.2/sascstdemodataProcess Inputs
The sourcemetadata
type refers to two data sets that contain the SDTM domain metadata,
source_tables and source_columns. Both data sets are stored in the
same library. Because the sample create_crtdds10_from_sdtm311.sas
driver program provided with the SAS Clinical Standards Toolkit references
a source CDISC SDTM 3.1.2 study, the source_tables data set contains
SDTM 3.1.2 metadata about each standard domain defined in the CDISC-SDTM 3.1.2 Implementation Guide and includes any
customizations that you have added. The source_columns type contains
similar metadata, but it is at the column level. This source metadata
is read from the
!sasroot/../../SASClinicalStandardsToolkitSDTM312/1.3/sample/cdisc-sdtm-3.1.2/sascstdemodata/metadata directory. This location is represented in the driver program by
the Srcmeta library name.
A source
study data set (source_study.sas7bdat) is required by this macro.
The following variables are required in this data set:
Process Outputs
The sourcedata
type is the library where the metadata files are created. These metadata
files are the data sets that constitute the SAS representation of
the CDISC CRT-DDS 1.0 standard. The create_crtdds10_from_sdtm311.sas
driver program creates 39 data sets. Most of these data sets have
zero observations because there is no default SDTM metadata source.
In the SAS Clinical Standards Toolkit sample study, these data sets
are written to the
!sasroot/../../SASClinicalStandardsToolkitCRTDDS10/1.3/sample/cdisc-crtdds-1.0/data directory. This location is represented in the driver program by
the srcdata library name.
Process Results
When the
driver program finishes running, the work._cstresults.sas7bdat data
set is created. This data set contains informational, warning, and
any error messages that were generated by the submitted driver program.
Because the create_crtdds10_from_sdtm311.sas sample SASreferences
data set does not include a results record, this example does not
save the process results data set after the SAS session ends.
Sample Driver Program: create_crtdds_define.sas
Overview
The create_crtdds_define.sas
driver program sets up the required environment variables and library
references to initiate the crtdds_write.sas macro. This macro reads
the 39 data sets that comprise the SAS representation of the CDISC
CRT-DDS 1.0 model, and converts that information to the required define.xml
structure. If source metadata or data are missing, then empty elements
and attributes are not created in the define.xml file. The inputs
and outputs are specified in the SASRferences data set. The following
table lists the optional parameters that can be set by the user when
submitting the macro:
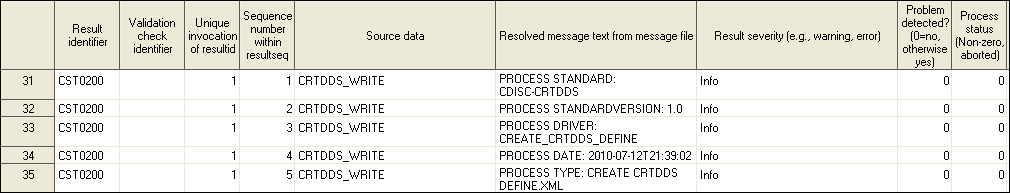
Parameters for the crtdds_write.sas Macro
%crtdds_write(_cstCreateDisplayStyleSheet=1, _cstOutputEncoding=UTF-16,
_cstResultsOverrideDS=&_cstResultsDS);
In this
example, a default style sheet is generated in the same directory
as the XML output based on the information in the SASReferences data
set. XML encoding is set to UTF-16, and process results are written
to the default &_cstResultsDS data set.
The create_crtdds_define.sas
driver program is ready to run on any of the CDISC SDTM sample studies.
The driver program can be run interactively or in batch.
!sasroot/../SASClinicalStandardsToolkitCRTDDS10/1.3/sample/cdisc-crtdds-1.0/programs/create_crtdds_define.sas!sasroot/../../SASClinicalStandardsToolkitCRTDDS10/1.3/sample/cdisc-crtdds-1.0/programs/create_crtdds_define.sasMultiple
tasks can be executed in any SAS Clinical Standards Toolkit driver
program. The create_crtdds_define.sas driver program calls both the
crtdds_write macro to create the define.xml file, and the crtdds_xmlvalidate
macro to validate the syntax of the generated define.xml file. For
more information about the crtdds_xmlvalidate macro, see Validation of XML-Based Standards.
The SASReferences Data Set
As a part
of each SAS Clinical Standards Toolkit process setup, a valid SASReferences
data set is required. It can be modified to point to study-specific
files. For an explanation of the SASReferences data set, see SASReferences File.
In the
SASReferences data set, there are two input file references and three
output references that are key to successful completion of the create_crtdds_define.sas
driver program. The following table lists these files and data sets,
and they are discussed in separate sections. In the sample create_crtdds_define.sas
driver program, the following values are set for &studyRootPath
and &studyOutputPath and are specific to a SAS release.
Process Inputs
Use of
the control library name that points to the path in the &workpath
macro variable illustrates a technique of documenting the derivation
of the SASReferences data set in the SAS Work library. The driver
program initiates the macro variable &workpath with the following
SAS code:
%let workPath=%sysfunc(pathname(work));
The sourcedata
type is the library that contains the 39 data sets that might have
been populated by the create_crtdds10_from_sdtm311.sas driver program.
These metadata files are the data sets that constitute the SAS representation
of the CDISC CRT-DDS 1.0 standard. In the SAS Clinical Standards Toolkit
sample study, these data sets are read from the
!sasroot/../../SASClinicalStandardsToolkitCRTDDS10/1.3/sample/cdisc-crtdds-1.0/data directory. This location is represented in the driver program by
the Srcdata library name.
Process Outputs
The externalxml
type refers to the define.xml file. This file is accessed in the driver
program from the extxml filename statement, and is written to the
!sasroot/../../SASClinicalStandardsToolkitCRTDDS10/1.3/sample/cdisc-crtdds-1.0/sourcexml directory.
The referencexml
type can serve as either an input or output file reference. Because
the path and filename are not provided, the crtdds_write macro interprets
the _cstCreateDisplayStyleSheet=1 parameter to use the default style
sheet that is provided by SAS Clinical Standards Toolkit in the Global
Library. Had a path and filename been provided, the referencexml type
would serve as an output file reference for the crtdds_write macro
to copy the default style sheet from the Global Library to the path
and filename that were specified. The results type refers to the write_results
data set that documents the create define process results. In the
SAS Clinical Standards Toolkit CDISC CRT-DDS folder hierarchy, this
information is written to the
!sasroot/../../SASClinicalStandardsToolkitCRTDDS10/1.3/sample/cdisc-crtdds-1.0/results directory.