Managing Controlled Terminology
Overview: Managing Controlled Terminology
SAS Clinical Data Integration
enables you to manage controlled terminology. Controlled
terminology is a set of possible values for something.
For example, controlled terminology for the valid values of yes and
no could be expressed as (1-Yes, 2-No).
A terminology
table is a SAS data set that contains controlled terminology
data. SAS Clinical Standards Toolkit provides CDISC terminology tables.
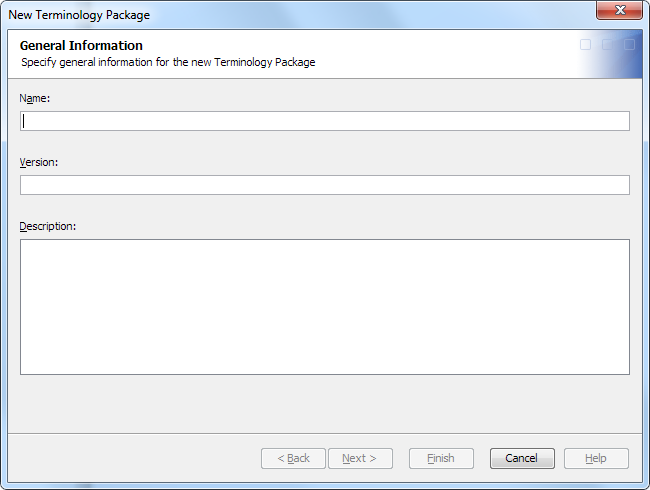
A terminology
package is a group of terminology tables. The data standards
administrator creates terminology packages. The data standards administrator
manages the granularity of the terminology and the groups to which
the terminologies are available. For example, the following is the
granularity of the terminology and the group to which it is available:
-
a study or submission
-
the transformations that use the controlled terminology
When a new study or
submission is created, the trial manager selects the terminology package
to use for the study or submission. This information is used by the
CDISC-SDTM Compliance transformation and the CDISC-Define Creation
transformation.
If multiple terminology
data sets are specified for a study or submission, changing the order
of the terminology data sets affects the order in which the terminology
tables are applied during a transformation. If a controlled term is
defined several times, the first value found is the value used.
Importing Terminology Data Sets
To manage controlled
terminology, you import CDISC terminology data sets from SAS Clinical
Standards Toolkit. A wizard imports the controlled terminology and
creates the associated terminology data set.
After a terminology
data set is imported, you can verify that the import was successful.
You can open, delete, or rename the terminology data sets using SAS
Data Integration Studio. For more information, see SAS
Data Integration Studio: User's Guide or the SAS Data
Integration Studio online Help.
You can create, rename,
or change the order in which the terminology tables in the data set
are applied during a transformation.
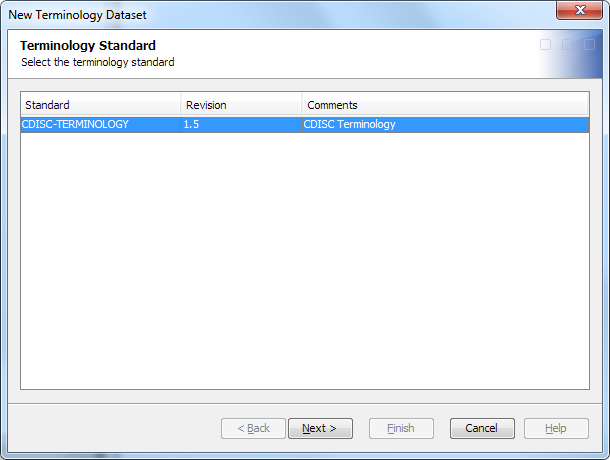
Import a Terminology Data Set
To import a terminology
data set, perform the following steps:
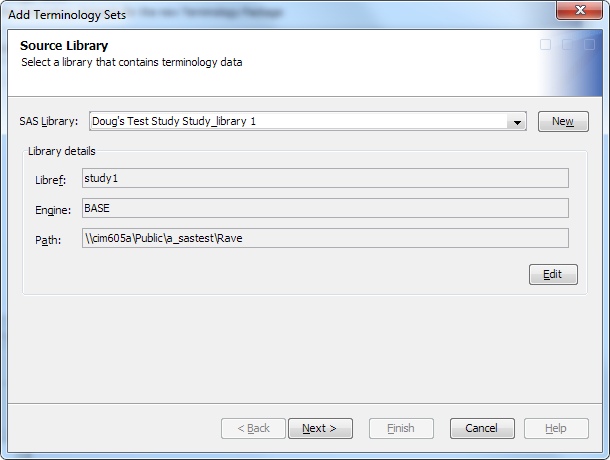
-
From the SAS Library drop-down list, select the library that contains the imported terminology table or create a library, or click New to create one.For information about using the New Library Wizard to create a library, see the SAS Data Integration Studio: User's Guide or the SAS Data Integration Studio online Help.Note: The selected library must have Create permission for the user who is currently logged on.
Copyright © SAS Institute Inc. All rights reserved.