Managing the Codelist Table in a SAS Clinical Data Integration Study
Overview: Managing the Codelist Table in a SAS Clinical Data Integration Study
When you import a codelist
table, SAS Clinical Data Integration imports the codelist table only
for the study versions that currently have or that could have (in
the future) enrolled subjects. These study versions are considered non-retired versions.
A non-retired version
is the desired version set for a specific study environment. This
version set contains all study versions which, for that study environment,
contain at least one enrolled subject. The most recent version for
an environment or site is included in the set for any environment
or site in that study. As a result, the most recent version is always
available for the forward migration of subject data, even if no subjects
are currently enrolled in the most recent version.
Limiting the codelist
table in this way ensures that the codelist table does not include
data for versions that will never be referenced.
The structure of a SAS
data set required to store Medidata Rave codelist table information
is the same across all versions. Therefore, the data in a SAS data
set for a codelist table can be reimported without data mismatch or
metadata mismatch.
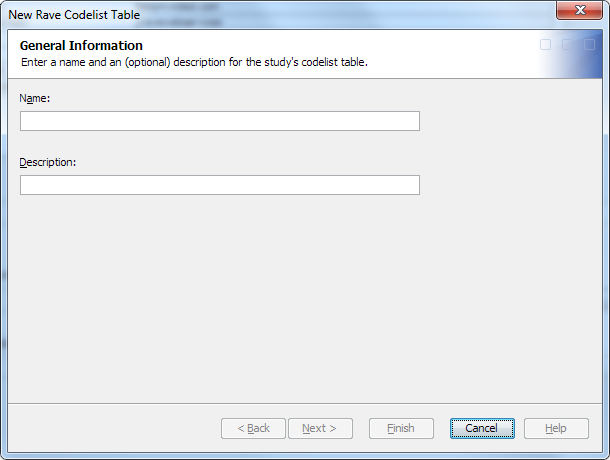
Create the Codelist Table for a SAS Clinical Data Integration Study
To create the codelist
table for a SAS Clinical Data Integration study, perform the following
steps:
-
For information, see Display the Medidata Rave Properties.
-
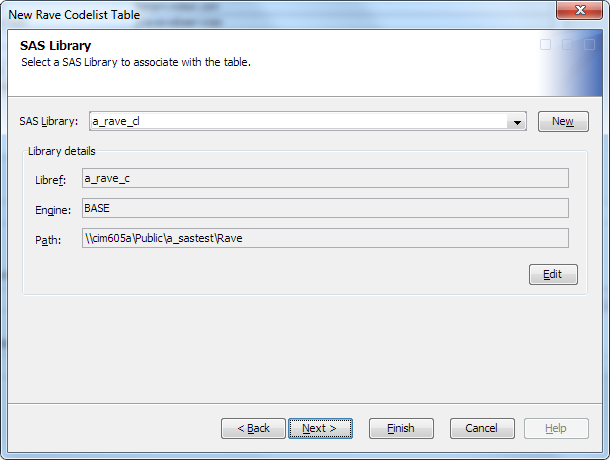
Using the standard SAS Data Integration Studio library controls, select a library, or create a library definition.Note: You must have Create access permission to the library that you select.For help with using these controls, see the SAS Data Integration Studio: User's Guide or the SAS Data Integration Studio online Help.
-
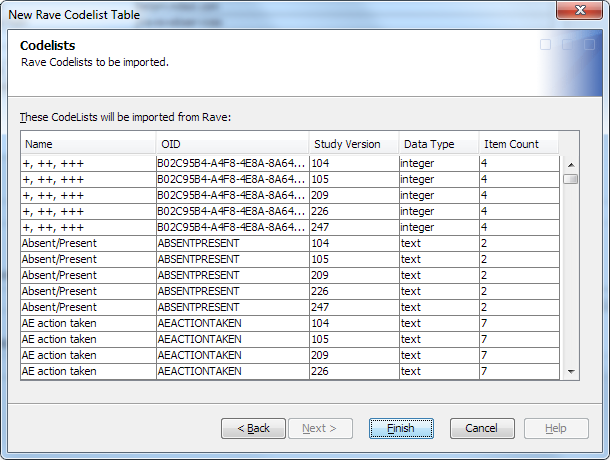
The Codelists page appears.The table lists the codelist tables that are registered to active Medidata Rave studies to which the SAS Clinical Data Integration study is mapped.Each row in the table represents one declared codelist table to import. The same codelist table can appear multiple times, once for each study version.
Remove the Codelist Table from a SAS Clinical Data Integration Study
To remove the codelist
table from a study, perform the following steps:
-
For information, see Display the Medidata Rave Properties.The Codelist Table field displays the name of the codelist table associated with the study.
Copyright © SAS Institute Inc. All rights reserved.