Managing Controlled Terminology
Overview: Managing Controlled Terminology
SAS Clinical Data Integration
enables you to manage controlled terminology. Controlled
terminology is a set of possible values for something.
For example, controlled terminology for the valid values of yes and
no could be expressed as (1-Yes, 2-No).
A terminology
table is a SAS data set that contains controlled terminology
data. SAS Clinical Standards Toolkit provides CDISC terminology tables.
A terminology
package is a group of terminology tables. The data standards
administrator creates terminology packages. The data standards administrator
manages the granularity of the terminology and the groups to which
the terminologies are available. For example, the following is the
granularity of the terminology and the group to which it is available:
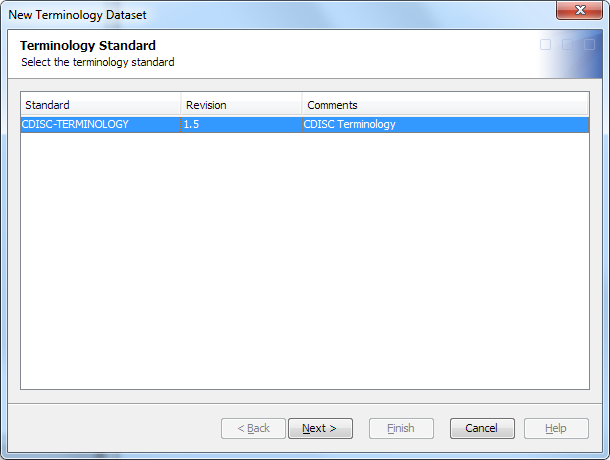
Importing Terminology Data Sets
To manage controlled
terminology, you import CDISC terminology data sets from SAS Clinical
Standards Toolkit. A wizard imports the controlled terminology and
creates the associated terminology data set.
After a terminology
data set is imported, you can verify that the import was successful.
You can open, delete, or rename the terminology data sets using SAS
Data Integration Studio. For more information, see SAS
Data Integration Studio: User's Guide or the SAS Data
Integration Studio online Help.
You can create, rename,
or change the order in which the terminology tables in the data set
are applied during a transformation.
Note: You must have the appropriate
permissions to import terminology data sets. For more
information, see Adding Users to the Clinical Administrators Group.
Import a Terminology Data Set
Copyright © SAS Institute Inc. All rights reserved.