Importing Data Standards Metadata
Overview
You define a study relative
to a particular data standard, such as CDISC-SDTM or a data standard
from your company, by importing the data standard from the SAS Clinical
Standards Toolkit.
If you want to use a
CDISC standard, import data standard metadata from the SAS Clinical
Standards Toolkit. Importing the metadata enables you to update your
environment with new releases of data standards from CDISC. CDISC
data standards are provided with the SAS Clinical Standards Toolkit.
For more information, see the SAS Clinical Standards Toolkit:
User's Guide.
After importing a data
standard that supports domain templates, column groups, and validation
data sets, these items are displayed in the Data Standards folder in the Clinical Administration tree.
For example, if you select the CDISC-SDTM data standard type and version
3.1.2, the domain templates (SDTM domains), column groups (SDTM classes),
and validation data sets (compliance checks) are displayed in a folder
that you specify in the Import wizard.
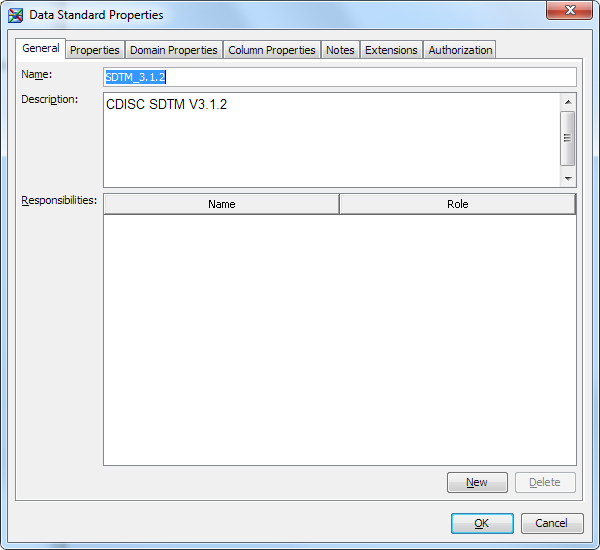
By default, an imported
data standard has a status of Inactive. This status enables you or
a data standards administrator to review the template and make changes
before releasing it for general use. When you or the data standards
administrator is satisfied with the template, you can change its status
to Active, which makes the template available for general use. For more
information, see Make a Data Standard Available for General Use.
Note: You must have appropriate
permissions to view the Clinical Administration tree. For more information, see Adding Users to the Clinical Administrators Group.
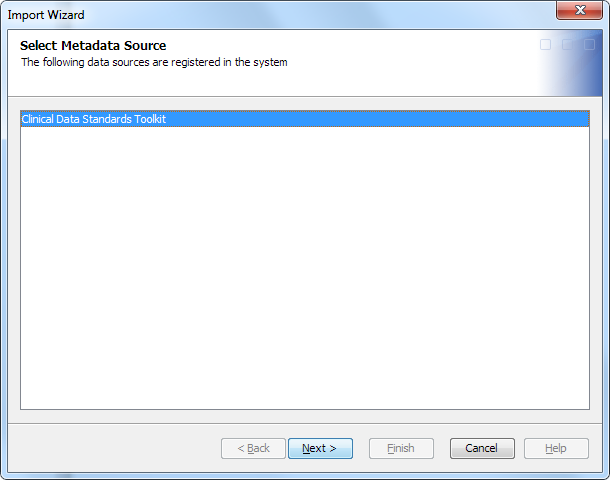
Import Data Standards Metadata
To import data standards
metadata, perform the following steps:
-
The CDISC standards that are listed are in the form of the standard short name, followed by the standard version number. For example, for SDTM 3.1.2, SDTM is the standard short name, and 3.1.2 is the standard version number). Each standard listing might also include a revision number that indicates the SAS Clinical Standards Toolkit version from which the standard definition was taken (for example, SDTM 3.1.2 (Revision 1.4).
-
-
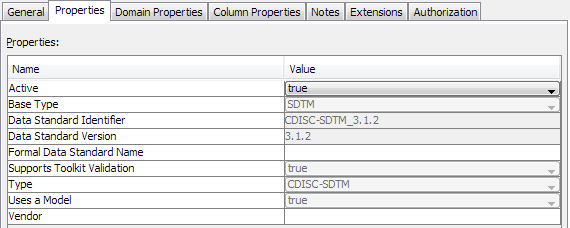
The values in the list are set up by the SAS Clinical Data Integration system administrator. These values are important when using the data standards metadata in the SAS Clinical Standards Toolkit. If the required type is not in the list, you should contact the system administrator to add the type, especially if the data standard uses the SAS Clinical Standards Toolkit. For more information about adding types, see Customizing Data Standard Properties.
-
(Optional) On the Verify Domain Column Metadata page, review the columns that are defined in the data standard, and then click Next.Note: This information is provided only as a reference. It enables you to review the metadata before storing it. You cannot make changes to this information. If you see problems in the metadata, then contact whomever is responsible for registering the data standard in the SAS Clinical Standards Toolkit.