SASReferences
Each SAS Clinical Standards
Toolkit process (for example, a primary task or action such as validating
source data against a SAS Clinical Standards Toolkit standard) requires
using a SASReferences data set. The SASReferences data set identifies
all of the inputs required and the outputs that are created by the
process. Each process might have its own unique SASReferences data
set.
SASReferences File, describes
the content and usage of SASReferences data sets.
The following table
identifies and describes each column within a SASReferences data set:
|
Column Name
|
Column Length
|
Description
|
|---|---|---|
|
standard
|
($20)
|
Standard name. This
value should match the
standard field
in the Standards data set in global standards library directory/metadata and
in other metadata files referenced in SASReferences (for example,
CDISC SDTM and CDISC CRT-DDS). This column is required.
|
|
standardversion
|
($20)
|
Specific version of
a standard. This value should match one of the standardversion values
associated with the
standard field
in the Standards data set in global standards library directory/metadata and
in other metadata files referenced in SASReferences (for example,
3.1.1 or 1.0). This column is required.
|
|
type
|
($40)
|
The type of input and
output data or metadata. This is a predefined set of values that are
documented in the
global standards library directory/standards/cst-framework-1.7/control/standardlookup data
set. These values are also itemized in SAS Clinical Standards Toolkit SASReferences Type and Subtype Values. This column is required.
|
|
subtype
|
($40)
|
The specific subtype
within type of input and output data or metadata. This is a predefined
set of values that are documented in the
global standards library directory/standards/cst-framework-1.7/control/standardlookup data
set. These values are also itemized in SAS Clinical Standards Toolkit SASReferences Type and Subtype Values. This column is optional, depending on type.
|
|
SASref
|
($8)
|
The SAS libref or fileref
that references the library or file in the SAS Clinical Standards
Toolkit SAS process. This value should match the value of sasref that
is used in any other associated metadata files (for example, in the
Source Columns data set, the value is type=srcmeta). This column is
required. It must conform to SAS libref or fileref naming conventions.
|
|
reftype
|
($8)
|
The reference type.
This column is required. Valid values are libref and fileref.
|
|
iotype
|
($8)
|
The input/output type
(input, output, or both) of the entity. Entities defined as “input”
or “both” must exist and be accessible. If not, calls
to the %CSTUTILVALIDATESASREFERENCES macro report an error condition
and halt the process.
|
|
filetype
|
($8)
|
The file type (folder,
dataset, catalog, or file).
|
|
allowoverwrite
|
($1)
|
Allow the file to be
overwritten (Y/N), for files with an iotype value of “output”
or “both”.
|
|
relpathprefix
|
($41)
|
The relative path prefix
(for example, rootpath, studylibraryrootpath, or &mypath). If
non-null, the value of the path is assumed to be relative to the resolved
relpathprefix. The reserved values rootpath and studylibraryrootpath
have special significance: they instruct the SAS Clinical Standards
Toolkit to use the standard-specific values for these columns in the
global standards library directory/metadata/standards.sas7bdat data
set.
|
|
path
|
($2048)
|
The path of the library
or the path portion of the file reference. If you want to use the
default value for a standard, standardversion, type, or subtype, then
leave the path blank. The value is added to the &_cstSASRefs working
version of the SASReferences data set from the standard-specific StandardSASReferences
data set. Specific paths should be provided for any type or subtype
that is study- or run-specific. Paths might be relative to an environment
variable (for example, !sasroot)
or to a SAS macro variable (for example, &studyRootPath).
|
|
order
|
(8.)
|
Processing or concatenation
order within type. If this value exists, then it should be a positive
integer with no duplicates within type. This column is optional, depending
on type. The order should be specified if one of these is true:
|
|
memname
|
($48)
|
The name of a specific
SAS file (data set or catalog) or file that is not created by SAS
(for example, properties or an XML file). The memname column should
be blank for library references. This column is optional, depending
on type. As a general rule, memname should be provided if the path
is provided, except where individual file references are not appropriate
(for example, type=autocall and type=sourcedata). If you want to use
the default value for a standard, standardversion, type, or subtype,
then leave memname blank. The value is added to the &_cstSASRefs
working version of the SASReferences data set from the standard-specific
StandardSASReferences data set. The file suffix for SAS files is optional.
|
|
comment
|
($200)
|
Explanatory comments.
This column is optional.
|
The following display
shows some information in a typical SAS Clinical Standards Toolkit
SASReferences data set:
A Sample SASReferences Data Set
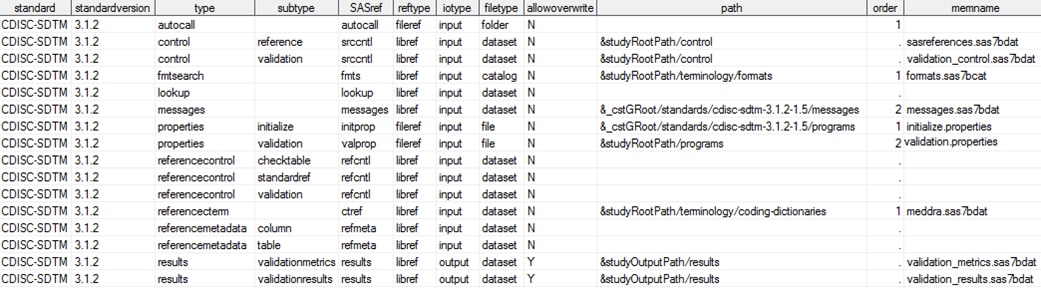
From this display, you
can see that the data set contains information about types of data
and metadata and where they are located. The SAS Clinical Standards
Toolkit imposes a rigid, minimum SASReferences file structure. All columns
defined in SASReferences Data Set Structure are expected; additional columns are allowed. No changes to column attributes are allowed (for example,
changing column lengths).
Note: SASReferences data sets from
the SAS Clinical Standards Toolkit releases prior to version 1.5 can
be used in version 1.7
if they do not include any of the columns added in version 1.5 (iotype,
filetype, allowoverwrite, and relpathprefix).
Copyright © SAS Institute Inc. All Rights Reserved.