| The SEQDESIGN Procedure |
| Table Output |
For each design, the SEQDESIGN procedure displays the "Design Information," "Method Information," and "Boundary Information" tables by default.
Boundary Information
The "Boundary Information" table displays the following information at each stage:
proportion of information
actual information level, if the maximum information is either specified or derived
alternative references with the specified statistic scale. If a
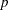 -value scale is specified, the standardized
-value scale is specified, the standardized 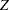 scale is used.
scale is used. boundary values with the specified statistic scale to reject or accept the null hypothesis
Note that implicitly, the boundary information table also contains variables for the boundary scale, stopping criterion, and type of alternative hypothesis. That is, if an ODS statement is used to save the table, the data set also contains the variables _Scale_ for the boundary scale, _Stop_ for the stopping criterion, and _ALT_ for the type of alternative hypothesis.
Design Information
The "Design Information" table displays the design specifications and derived statistics. The derived Max Information (Percent Fixed-Sample) is the maximum information for the sequential design in percentage of the corresponding fixed-sample information.
The Null Ref ASN (Percent Fixed-Sample) is the average sample number (expected sample size for nonsurvival data or expected number of events for survival data) required under the null hypothesis for the group sequential design in percentage of the corresponding fixed-sample design. Similarly, the Alt Ref ASN (Percent Fixed-Sample) is the average sample number required under the alternative reference for the group sequential design in percentage of the corresponding fixed-sample design.
If both the maximum information (MAXINFO= option) and the alternative reference 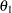 (ALTREF= option) are specified, then either the ALPHA= option is used to derive the Type II error probability
(ALTREF= option) are specified, then either the ALPHA= option is used to derive the Type II error probability 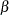 (BOUNDARYKEY=ALPHA) or the BETA= option is used to derive the Type I error probability
(BOUNDARYKEY=ALPHA) or the BETA= option is used to derive the Type I error probability  (BOUNDARYKEY=BETA).
(BOUNDARYKEY=BETA).
Error Spending Information
The "Error Spending Information" table displays the following information at each stage:
proportion of information
actual information level, if the maximum information is either specified or derived
cumulative error spending for each boundary
Method Information
The "Method Information" table displays detailed method information for the design. For each boundary, it displays the following:
the group sequential method used
the
 or
or 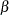 errors
errors the specified parameter
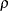 , if an error spending function is used
, if an error spending function is used the specified parameters
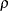 and
and  with the derived critical value
with the derived critical value 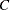 , if a unified family method is used
, if a unified family method is used the alternative reference
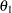 , if either the ALTREF= or the MAXINFO= option is specified
, if either the ALTREF= or the MAXINFO= option is specified the derived drift parameter,
 , where
, where 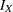 is the maximum information and
is the maximum information and 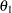 is the alternative reference
is the alternative reference
Note that the alternative references are displayed with the MLE scale in the "Method Information" table. In contrast, the alternative references in the "Boundary Information" table are displayed with the specified statistic scale (if the 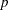 -value scale is not specified) or the standardized
-value scale is not specified) or the standardized 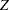 scale (if the
scale (if the 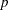 -value scale is specified).
-value scale is specified).
Powers and Expected Sample Sizes
The "Powers and Expected Sample Sizes" table displays the following information under each of the specified hypothetical references 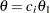 , where
, where 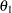 is the alternative reference and
is the alternative reference and 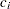 are values specified in the CREF= option.
are values specified in the CREF= option.
coefficient
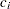 for the hypothetical references. The value
for the hypothetical references. The value 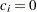 corresponds to the null hypothesis and
corresponds to the null hypothesis and  corresponds to the alternative hypothesis
corresponds to the alternative hypothesis power
expected sample size, as percentage of fixed-sample size
For a one-sided design, the power and expected sample sizes under the hypothetical references 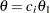 are displayed.
are displayed.
For a two-sided symmetric design, the power and expected sample sizes under each of the hypothetical references 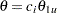 are displayed, where
are displayed, where 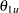 is the upper alternative reference.
is the upper alternative reference.
For a two-sided asymmetric design, the power and expected sample sizes under each of the hypothetical references  and
and 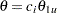 are displayed, where
are displayed, where 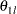 and
and 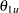 are the lower and upper alternative references, respectively.
are the lower and upper alternative references, respectively.
For a two-sided design, the power is the probability of correctly rejecting the null hypothesis for the correct alternative. Thus, under the null hypothesis, the displayed power corresponds to a one-sided Type I error probability level—that is, the lower  level or the upper
level or the upper  level.
level.
The expected sample size as a percentage of the corresponding fixed-sample design is
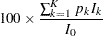 |
where 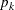 is the stopping probability at stage
is the stopping probability at stage 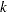 ,
,  is the expected information level, and
is the expected information level, and  is the information level for the fixed-sample design.
is the information level for the fixed-sample design.
Sample Size Summary
When you use the SAMPLESIZE statement with the SEQDESIGN procedure, the "Sample Size Summary" table displays parameters for the sample size computation. It also displays the expected sample sizes or numbers of events for the model under both the null and alternative hypotheses.
The expected sample size is the average sample size
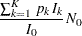 |
where 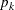 is the stopping probability at stage
is the stopping probability at stage 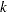 ,
,  is the expected information level,
is the expected information level,  is the information level for the fixed-sample design, and
is the information level for the fixed-sample design, and 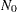 is the sample size for the fixed-sample design.
is the sample size for the fixed-sample design.
The expected number of events is the average number of events
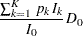 |
where 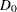 is the fixed-sample number of events for the model.
is the fixed-sample number of events for the model.
Sample Size Information
The "Sample Sizes (N)" table displays the required sample sizes and information levels at each stage, in both fractional and integer numbers. The derived fractional sample sizes are under the heading "Fractional N." These sample sizes are rounded up to integers under the heading "Ceiling N." The matched integer sample sizes are also displayed for two-sample tests.
The "Required Number of Events (D)" table displays the required number of events required and information level at each stage.
The "Number of Events (D) and Sample Sizes (N)" table displays the number of events and sample size required at each stage with the study time. The derived times under the heading "Fractional Time" are not integers. These times are rounded up to integers under the heading "Ceiling Time."
Stopping Probabilities
The "Expected Cumulative Stopping Probabilities" table displays the following information under each of the specified hypothetical references 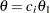 , where
, where 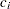 are values specified in the CREF= option, and
are values specified in the CREF= option, and 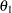 is the alternative reference:
is the alternative reference:
coefficient
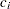 for the hypothetical references. The value
for the hypothetical references. The value 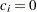 corresponds to the null hypothesis, and
corresponds to the null hypothesis, and  corresponds to the alternative hypothesis
corresponds to the alternative hypothesis expected stopping stage
source of the stopping probability: reject
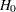 (with STOP=REJECT or STOP=BOTH), accept
(with STOP=REJECT or STOP=BOTH), accept 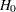 (with STOP=ACCEPT or STOP=BOTH), or either reject or accept
(with STOP=ACCEPT or STOP=BOTH), or either reject or accept 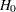 (with STOP=BOTH)
(with STOP=BOTH) expected cumulative stopping probabilities at each stage
For a one-sided design, the expected cumulative stopping probabilities under the hypothetical references 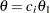 are displayed.
are displayed.
For a two-sided design, the expected cumulative stopping probabilities under each of the hypothetical references  and
and 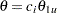 are displayed, where
are displayed, where 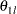 and
and 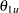 are the lower and upper alternative references, respectively.
are the lower and upper alternative references, respectively.
Note that for a symmetric two-sided design, only the expected cumulative stopping probabilities under the hypothetical references 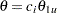 are derived.
are derived.
The expected stopping stage is given by 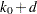 , where the integer
, where the integer 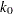 and the fraction
and the fraction 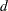 (
( ) are derived from the expected information level equation
) are derived from the expected information level equation
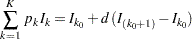 |
where 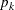 is the stopping probability at stage
is the stopping probability at stage 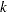 .
.
For equally spaced information levels, the expected stopping stage is reduced to the weighted average
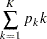 |
Copyright © SAS Institute, Inc. All Rights Reserved.