| The SEQDESIGN Procedure |
| One-Sided Fixed-Sample Tests in Clinical Trials |
A one-sided test has either an upper (greater) or a lower (lesser) alternative. This section describes one-sided tests with upper alternatives only. Corresponding results for one-sided tests with lower alternatives can be derived similarly.
For a one-sided test of 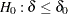 with an upper alternative
with an upper alternative  , an equivalent null hypothesis is
, an equivalent null hypothesis is  with an upper alternative
with an upper alternative 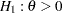 , where
, where 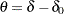 . A fixed-sample test rejects
. A fixed-sample test rejects 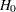 if the standardized test statistic
if the standardized test statistic  , where
, where  is the sample estimate of
is the sample estimate of 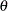 and
and  is the critical value.
is the critical value.
The 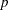 -value of the test is given by
-value of the test is given by  , and the hypothesis
, and the hypothesis 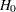 is rejected if the
is rejected if the 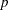 -value is less than
-value is less than  . An upper
. An upper 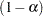 confidence interval has the lower limit
confidence interval has the lower limit
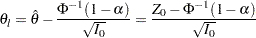 |
The hypothesis 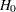 is rejected if the confidence interval for the parameter
is rejected if the confidence interval for the parameter 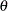 does not contain zero—that is, if the lower limit
does not contain zero—that is, if the lower limit 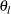 is greater than
is greater than 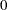 .
.
With an alternative reference  ,
, 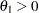 , a Type II error probability is defined as
, a Type II error probability is defined as
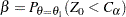 |
which is equivalent to
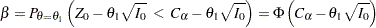 |
Thus,  . Then, with
. Then, with 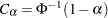 ,
,
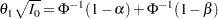 |
The drift parameter  can be computed for specified
can be computed for specified  and
and 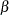 and the maximum information is given by
and the maximum information is given by
 |
If the maximum information is available, then the required sample size can be derived. For example, in a one-sample test for the mean with a specific standard deviation  , the sample size
, the sample size  required for the test is
required for the test is
 |
On the other hand, if the alternative reference  , standard deviation
, standard deviation  , and sample size
, and sample size  are all specified, then
are all specified, then  can be derived for a given
can be derived for a given 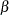 and, similarly,
and, similarly, 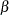 can be derived for a given
can be derived for a given  .
.
With an alternative reference  ,
, 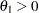 , the power
, the power 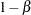 is the probability of correctly rejecting the null hypothesis
is the probability of correctly rejecting the null hypothesis 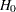 at
at 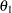 :
:
 |
Superiority Trials
A superiority trial that tests the response to a new drug is clinically superior to a comparative placebo control or active control therapy. If a positive value indicates a beneficial effect, a test for superiority has
 |
where 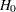 is the hypothesis of nonsuperiority and
is the hypothesis of nonsuperiority and 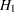 is the alternative hypothesis of superiority.
is the alternative hypothesis of superiority.
The superiority test rejects the hypothesis 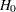 and declares superiority if the standardized statistic
and declares superiority if the standardized statistic 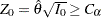 , where the critical value
, where the critical value  .
.
For example, if 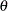 is the response difference between the treatment and placebo control groups, then a superiority trial can be
is the response difference between the treatment and placebo control groups, then a superiority trial can be
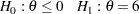 |
with a Type I error probability level 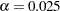 and a power
and a power 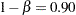 at
at  .
.
Noninferiority Trials
A noninferiority trial does not compare the response to a new treatment with the response to a placebo. Instead, it demonstrates the effectiveness of a new treatment compared with that of a nonexisting placebo by showing that the response of a new treatment is not clinically inferior to the response of a standard therapy with an established effect. That is, this type of trial attempts to demonstrate that the new treatment effect is not worse than the standard therapy effect by an acceptable margin. These trials are often performed when there is an existing effective therapy for a serious disease, and therefore a placebo control group cannot be ethically included.
It can be difficult to specify an appropriate noninferiority margin. One practice is to choose with reference to the effect of the active control in historical placebo-controlled trials (Snapinn 2000, p. 20). With this practice, there is some basis to imply that the new treatment is better than the placebo for a positive noninferiority trial.
If a positive value indicates a beneficial effect, a test for noninferiority has a null hypothesis 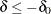 and an alternative hypothesis
and an alternative hypothesis 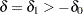 , where
, where 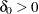 is the specified noninferiority margin.
is the specified noninferiority margin.
An equivalent test has
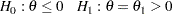 |
where the parameter  ,
, 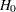 is the null hypothesis of inferiority, and
is the null hypothesis of inferiority, and 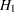 is the alternative hypothesis of noninferiority,
is the alternative hypothesis of noninferiority,
The noninferiority test rejects the hypothesis 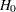 and declares noninferiority if the standardized statistic
and declares noninferiority if the standardized statistic 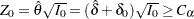 , where the critical value
, where the critical value  .
.
For example, if 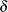 is the response difference between the treatment and active control groups and
is the response difference between the treatment and active control groups and 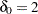 is the noninferiority margin, then a noninferiority trial with a power
is the noninferiority margin, then a noninferiority trial with a power 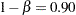 at
at 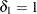 might be
might be
 |
where  .
.
Copyright © SAS Institute, Inc. All Rights Reserved.