CDISC Dataset-XML
Overview
CDISC Dataset-XML defines
a standard format for transporting tabular data in XML between any
two entities based on CDISC ODM XML. In addition to supporting the
transport of data sets as part of a submission to the FDA, Dataset-XML
can be used to exchange data between two parties. For example, the
Dataset-XML data format can be used by a CRO to transmit SDTM or ADaM
data sets to a sponsor organization. Dataset-XML supports SDTM, ADaM,
and SEND data sets but can also be used to exchange any other type
of tabular data set.
Dataset-XML and Define-XML
The metadata for a data
set in a Dataset-XML file must conform to the Define-XML standard.
Each Dataset-XML file contains data for a single data set, but a single
Define-XML file describes all of the data sets included in the folder.
Both Define-XML 1.0 and Define-XML 2.0 are supported for use with
Dataset-XML.
Creating Dataset-XML Files from SAS Data Sets: %DATASETXML_WRITE Macro
The %DATASETXML_WRITE
macro creates a Dataset-XML file from a SAS data set or from a library
of SAS data sets.
Here is an example:
libname srcdata "&studyRootPath/data"; filename srcmeta "&studyRootPath/sourcexml/define.xml"; libname xmldata "&studyOutputPath/sourcexml"; %datasetxml_write( _cstSourceLibrary=srcdata, _cstOutputLibrary=xmldata _cstSourceMetadataDefineFileRef=srcmeta, _cstCheckLengths=Y, _cstIndent=N, _cstZip=Y, _cstDeleteAfterZip=N );
In this example, the
Dataset-XML files are compressed into ZIP files, with one ZIP file
per Dataset-XML file. But, the Dataset-XML files are not deleted after
compression.
Instead of specifying
inputs (_cstSourceLibrary and _cstSourceMetadataDefineFileRef) and
outputs (_cstOutputLibrary) for the process with the parameters, you
can use the more traditional SASReferences data set. These different
ways of specifying parameters are demonstrated in two sample programs:
create_datasetxml_standalone.sas and create_datasetxml.sas. These
sample programs are located here:
sample study library directory/cdisc-datasetxml-1.0.0–1.7/programsNote: The create_datasetxml_standalone.sas
sample program does not use a SASReferences data set and writes reports
only in the SAS log file.
The Define-XML file
that describes the SAS data sets must contain metadata about all SAS
data sets and all variables to convert. The Dataset-XML files by themselves
do not have any information about the SAS data sets (name and label)
or the SAS variables (name, label, data type, length, and display
format). When the Dataset-XML file is converted back to SAS data sets,
this information must be provided by the Define-XML file.
Here is an example
of a Dataset-XML file:
<?xml version="1.0" encoding="UTF-8"?>
<ODM xmlns="http://www.cdisc.org/ns/odm/v1.3"
xmlns:data="http://www.cdisc.org/ns/Dataset-XML/v1.0"
ODMVersion="1.3.2" FileType="Snapshot" FileOID="cdisc01.AE"
PriorFileOID="www.cdisc.org.Studycdisc01-Define-XML_2.0.0"
CreationDateTime="2014-06-23T13:18:18"
data:DatasetXMLVersion="1.0.0">
<ClinicalData StudyOID="cdisc01"
MetaDataVersionOID="MDV.CDISC01.SDTMIG.3.1.2.SDTM.1.2">
<ItemGroupData ItemGroupOID="IG.AE" data:ItemGroupDataSeq="1">
...
<ItemData ItemOID="IT.AE.AETERM" Value="AGITATED"/>
Here is an example
of a Define-XML file:
<ODM ... >
<Study OID="cdisc01">
...
<MetaDataVersion OID="MDV.CDISC01.SDTMIG.3.1.2.SDTM.1.2"
Name="Study CDISC01, Data Definitions"
Description="Study CDISC01, Data Definitions"
def:DefineVersion="2.0.0" def:StandardName="SDTM-IG"
def:StandardVersion="3.1.2">
...
<ItemGroupDef OID="IG.AE"
Domain="AE" Name="AE" Repeating="Yes" IsReferenceData="No"
SASDatasetName="AE" Purpose="Tabulation"
def:Structure="One record per adverse event per subject"
def:Class="EVENTS" def:ArchiveLocationID="LF.AE">
...
<ItemRef ItemOID="IT.AE.AETERM" OrderNumber="6" Mandatory="Yes"/>
...
<ItemDef OID="IT.AE.AETERM" Name="AETERM" DataType="text" Length="25"
SASFieldName="AETERM">
A Dataset-XML file must
satisfy these requirements:
-
The ClinicalData attributes StudyOID and MetaDataVersionOID must be the same value as the corresponding OID attributes in the define.xml document.
-
The ItemGroupOID value must be the same value as the corresponding ItemGroup OID attribute in the define.xml document.
-
All ItemOID attributes in the ItemData elements must have values identical to the values of the corresponding ItemOID attributes in the ItemRef elements that are child elements of the corresponding ItemGroupDef element in the define.xml document.
It would be an error
to try to extract from the Dataset-XML file the SAS data set name
from an ItemGroup object identifier (ItemGroupOID=“IG.AE”).
It would also be an error to try to extract the variable name from
an object identifier (ItemOID=”IT.AE.AETERM”). There
is no requirement concerning the values of the identifiers.
SAS tables and columns
are matched to @SASDatasetName (or, if this value is not specified,
@Name) and @SASFieldName (or, if this value is not specified, @Name).
SASDatasetName and SASFieldName are optional but @Name is required.
So, @Name is always available.
If the ItemGroup or
ItemDef is not found, the XML is generated with this pattern for @ItemGroupOID
and @ItemOID:
ItemGroupOID = ”IG.<table>” ItemOID = “IT.<table>.<column>”
Although ItemGroupOID
and ItemOID are generated for missing ItemGroups or ItemDefs, it is
important to realize that this can lead to problems later when converting
Dataset-XML files to SAS data sets. For example, when converting a
Dataset-XML file into a SAS data set, ItemGroupOIDs or ItemOIDs that
cannot be matched in the corresponding Define-XML file can lead to
missing SAS data sets or missing SAS data set variables.
Warnings are written
to the SAS log file and the write_results data set in the results
folder.
Here is an example
of the SAS log file:
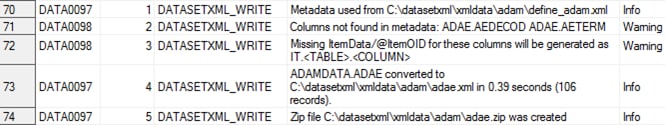
WARNING: [CSTLOGMESSAGE.DATASETXML_WRITE] Columns not found in metadata:
ADAE.AEDECOD ADAE.AETERM
WARNING: [CSTLOGMESSAGE.DATASETXML_WRITE] Missing ItemData/@ItemOID for column=AEDECOD
has been set to IT.ADAE.AEDECOD
WARNING: [CSTLOGMESSAGE.DATASETXML_WRITE] Missing ItemData/@ItemOID for column=AETERM
has been set to IT.ADAE.AETERM
The following display
shows an example of the write_results data set as created by the create_datasetxml.sas
sample program:
Example write_results Data Set
The @IsReferenceData
attribute in the Define-XML file determines whether the data set is
considered ReferenceData or ClinicalData. Here is an example:
<ReferenceData StudyOID="cdisc01"
MetaDataVersionOID="MDV.CDISC01.SDTMIG.3.1.2.SDTM.1.2">
<ItemGroupData ItemGroupOID="IG.TE" data:ItemGroupDataSeq="1">
<ItemData ItemOID="IT.STUDYID" Value="CDISC01"/>
<ItemData ItemOID="IT.TE.DOMAIN" Value="TE"/>
<ItemData ItemOID="IT.TE.ETCD" Value="EOS"/>
<ItemData ItemOID="IT.TE.ELEMENT" Value="End of Study"/>
<ItemData ItemOID="IT.TE.TESTRL" Value="Study Termination"/>
<ItemData ItemOID="IT.TE.TEDUR" Value="P1D"/>
</ItemGroupData>
<ClinicalData StudyOID="cdisc01"
MetaDataVersionOID="MDV.CDISC01.SDTMIG.3.1.2.SDTM.1.2">
<ItemGroupData ItemGroupOID="IG.AE" data:ItemGroupDataSeq="1">
<ItemData ItemOID="IT.STUDYID" Value="CDISC01"/>
<ItemData ItemOID="IT.AE.DOMAIN" Value="AE"/>
<ItemData ItemOID="IT.USUBJID" Value="CDISC01.100008"/>
<ItemData ItemOID="IT.AE.AESEQ" Value="1"/>
<ItemData ItemOID="IT.AE.AESPID" Value="1"/>
<ItemData ItemOID="IT.AE.AETERM" Value="AGITATED"/>
The _cstCheckLengths
macro parameter enables the %DATASETXML_WRITE macro to determine whether
the lengths defined in the metadata are long enough for character
data. This check is important to avoid data truncation problems when
importing the Dataset-XML files into SAS data set with the %DATASETXML_READ
macro. Warnings are written to the SAS log file and the write_results
data set in the results folder.
Here is an example
of the SAS log file:
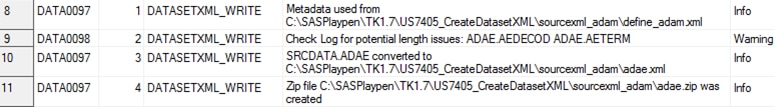
WARNING: [CSTLOGMESSAGE.DATASETXML_WRITE] Length too short: __ItemGroupOID=IG.ADAE
__ItemOID=IT.ADAE.AETERM Length=20 _valueLength=24 value=HEARTBURN-LIKE DYSPEPSIA
WARNING: [CSTLOGMESSAGE.DATASETXML_WRITE] Length too short: __ItemGroupOID=IG.ADAE
__ItemOID=IT.ADAE.AETERM Length=20 _valueLength=25 value=ACID REFLUX (OESOPHAGEAL)
WARNING: [CSTLOGMESSAGE.DATASETXML_WRITE] Length too short: __ItemGroupOID=IG.ADAE
__ItemOID=IT.ADAE.AEDECOD Length=20 _valueLength=32 value=Gastrooesophageal reflux disease
WARNING: [CSTLOGMESSAGE.DATASETXML_WRITE] Length too short: __ItemGroupOID=IG.ADAE
__ItemOID=IT.ADAE.AETERM Length=20 _valueLength=25 value=ACID REFLUX (OESOPHAGEAL)
The following display
shows an example of the write_results data set:
Example write_results Data Set
The %DATASETXML_WRITE
macro also checks that numeric variables in ADaM data sets that represent
date and time information have a DisplayFormat defined in the Define-XML
file.
Creating SAS Data Sets from Dataset-XML Files: %DATASETXML_READ Macro
The %DATASETXML_READ
macro creates a SAS data set or a library of SAS data sets from Dataset-XML
files.
Here is an example:
%datasetxml_read( _cstSourceDatasetXMLLibrary=dataxml, _cstOutputLibrary=sdtmdata, _cstSourceMetadataDefineFileRef=defxml, _cstDatetimeLength=64, _cstAttachFormats=Y, _cstNumObsWrite=10000 );
Instead of specifying
inputs (_cstSourceDatasetXMLLibrary and _cstSourceMetadataDefineFileRef)
and outputs (_cstOutputLibrary) for the process with parameters, you
can use the more traditional SASReferences data set. These different
ways of specifying parameters are demonstrated in two sample programs:
create_sas_from_datasetxml_standalone.sas and create_sas_from_datasetxml.sas.
These sample programs are located here:
sample study library directory/cdisc-datasetxml-1.0.0–1.7/programsNote: The create_sas_from_datasetxml_standalone.sas
sample program does not use a SASReferences data set and writes reports
only in the SAS log file.
The Define-XML file
that describes the Dataset-XML files must contain metadata information
about all Dataset-XML files and all variables to convert to SAS data
sets. The Dataset-XML files by themselves do not have any information
about the SAS data sets (name and label) or the SAS variables (name,
label, data type, length, and display format).
Character variables
that represent date- and time-related information in ADaM or SDTM
data conform to the ISO 8601 standard and do not have a length specified
in the Define-XML file. The _cstDateTimeLength parameter specifies
the length to use for these variables when they are converted to SAS
data sets. If the lengths of character variables are too short to
hold the data, warnings are written to the SAS log file and the read_results
data set in the results folder.
Here is an example
of the SAS log file:
WARNING: [CSTLOGMESSAGE.DATASETXML_READ] TRUNCATION occurred: Length=20 too short for
ItemGroupDataSeq=12 IT.ADAE.AETERM value=HEARTBURN-LIKE DYSPEPSIA (length=24)
WARNING: [CSTLOGMESSAGE.DATASETXML_READ] TRUNCATION occurred: Length=20 too short for
ItemGroupDataSeq=25 IT.ADAE.AETERM value=HEARTBURN-LIKE DYSPEPSIA (length=24)
WARNING: [CSTLOGMESSAGE.DATASETXML_READ] TRUNCATION occurred: Length=20 too short for
ItemGroupDataSeq=28 IT.ADAE.AETERM value=ACID REFLUX (OESOPHAGEAL)
WARNING: [CSTLOGMESSAGE.DATASETXML_READ] TRUNCATION occurred: Length=20 too short for
ItemGroupDataSeq=28 IT.ADAE.AEDECOD value=Gastrooesophageal reflux disease (length=32)
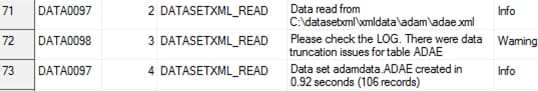
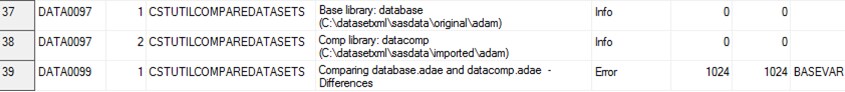
The following display
shows an example of the read_results data set as created by the create_sas_from_datasetxml.sas
sample program:
Example read_results Data Set
Inconsistencies between
the Dataset-XML file and the Define-XML file, which can lead to issues
with matching data to metadata, are written to the SAS log file and
the read_results data set in the results folder.
Here is an example
of the SAS log file:
WARNING: [CSTLOGMESSAGE.DATASETXML_READ] Items not found in metadata:
IT.ADAE.AEDECOD IT.ADAE.AETERM The following display
shows an example of the read_results data set:
Example read_results Data Set

In the following example,
the ADAE data set is created without the AETERM and AEDECOD variables,
as shown in this PROC COMPARE output:
Dataset Created Modified NVar NObs Label DATABASE.ADAE 23JUN14:09:01:29 23JUN14:09:01:29 61 106 Adverse Event Analysis Dataset DATACOMP.ADAE 02JUL14:12:58:14 02JUL14:12:58:14 59 106 Adverse Event Analysis Dataset Variables Summary Number of Variables in Common: 59. Number of Variables in DATABASE.ADAE but not in DATACOMP.ADAE: 2. Listing of Variables in DATABASE.ADAE but not in DATACOMP.ADAE Variable Type Length Label AETERM Char 200 Reported Term for the Adverse Event AEDECOD Char 200 Dictionary-Derived Term
The sample programs
create_sas_from_datasetxml.sas and create_sas_from_datasetxml_standalone.sas
also contain a call to the %CSTUTILCOMPAREDATASETS macro. This call
compares the original SAS data sets to the SAS data sets that were
created from the Dataset-XML files.
%cstutilcomparedatasets( _cstLibBase=sdtmdat0, _cstLibComp=sdtmdata, _cstCompareLevel=0, _cstCompOptions=%str(criterion=0.00000000000001), _cstCompDetail=Y );
For every SAS data
set that is compared, the macro reports the error code as returned
by PROC COMPARE. The following table shows the error codes:
Error Codes Returned by PROC COMPARE
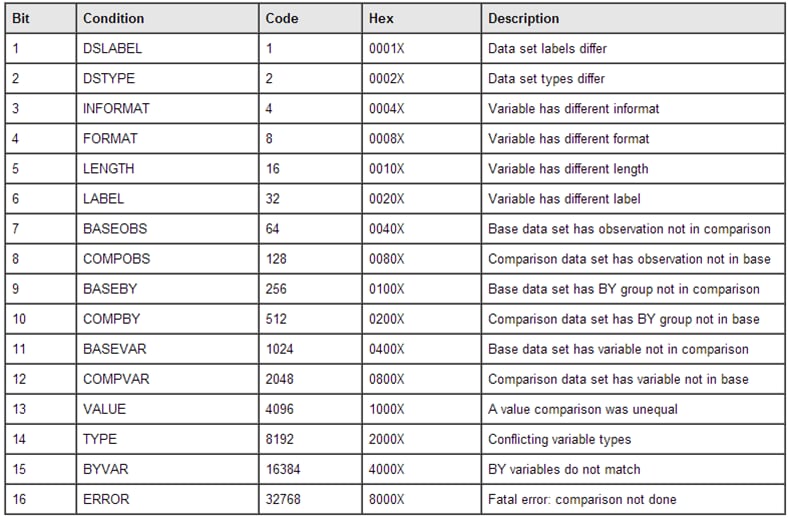
For example,
code=40
(8+32) indicates that a format and a label are different.
This message is written to the SAS log file:
WARNING: [CSTLOGMESSAGE.CSTUTILCOMPAREDATASETS] Comparing srcdata.adqs and
trgdata.adqs - Differences: FORMAT/LABEL (SysInfo=40)
When converting SAS
data sets to Dataset-XML and then converting back to SAS data sets,
here are difference to expect:
-
Date- and time-related columns do not have a length defined in the Define-XML metadata.Specify a length to assign in the macro (for example,
_cstdatetimeLength=64). The length is used to create the date- and time-related columns but can be different from the original lengths. -
SAS numeric variables are created with a length of 8 to avoid loss of precision, even when the original length or the length specified in the Define-XML file is less than 8.
-
Character variables (DataType=”text”) that do not have a length specified in the Define-XML file are created with a length of 200.
-
Small differences in precision can be expected around the machine precision for numeric variables that represent real numbers.
-
Character data that contains leading spaces or trailing spaces loses the leading and trailing spaces.
By specifying PROC COMPARE
options with the _cstCompOptions parameter, you can specify that the
comparison be less precise. For example,
_cstCompOptions=%str(criterion=0.00000000000001).
Lesser precision prevents differences close to machine precision from
being reported as errors.
The following display
shows an example of data set differences reported in the read_results
data set:
Example read_results Data Set
Copyright © SAS Institute Inc. All Rights Reserved.