Global Standards Library
The global standards
library is the metadata repository for the SAS Clinical Standards
Toolkit. By default, the global standards library contains the metadata
for the Framework module and the metadata for each data standard that
is provided with the SAS Clinical Standards Toolkit (such as the CDISC
SDTM 3.1.2 standard).
During the installation and configuration
of the SAS Clinical Standards Toolkit, you are prompted for the location
where the global standards library should be installed. The configuration
process creates a series of directories in this location.
-
logscontains the transactionlog data set used by the metadata management macros. For more information, see Metadata Management. -
metadatacontains data sets that have information about the registered standards. For more information, see Common Framework Metadata. -
schema-repositorycontains the schemas for XML-based standards that are supported. -
standardscontains a standard-specific directory hierarchy for each of the supported standards. -
xsl-repositorycontains directories and XSL files used in reading and writing XML files.
The
logs directory
contains one data set: transactionlog. This data set is populated
only by the metadata management macros. The data set can be updated
by one or more users depending on how the SAS Clinical Standards Toolkit
is implemented (file server installation or single installation on
a laptop). The data set contains metadata update information from
all users.
The
metadata directory
contains three data sets and one XML file: Standards, Standardlookup,
StandardSASReferences, and availabletransforms.xml. The Standards
data set has a list of the registered standards and basic information
relating to each standard.
The following display
shows the full content of the global standards library Standards data
set included with the SAS Clinical Standards Toolkit after a new installation
of the application:
Global Standards Library: Metadata Standards Data Set
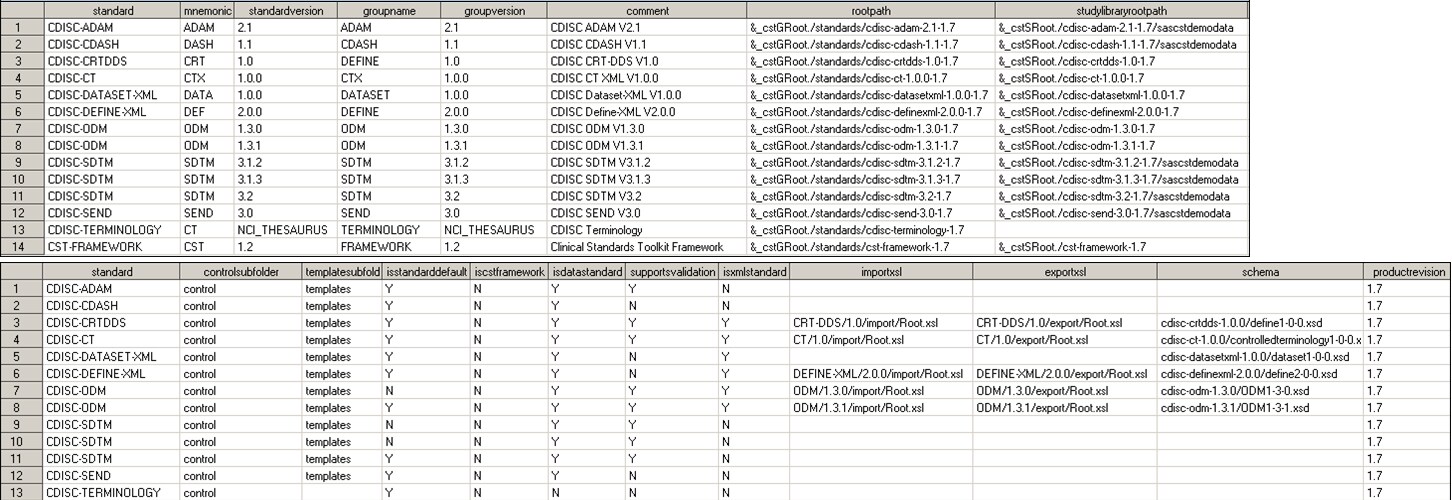
Note: The
&_cstGRoot directory
in the rootpath
column maps to the global standards library directory.
The StandardSASReferences
data set defines the typical inputs and outputs of SAS processes that
are associated with each standard.
The following display
shows some rows and columns:
Global Standards Library: Some Rows and Columns of the Metadata
StandardSASReferences Data Set
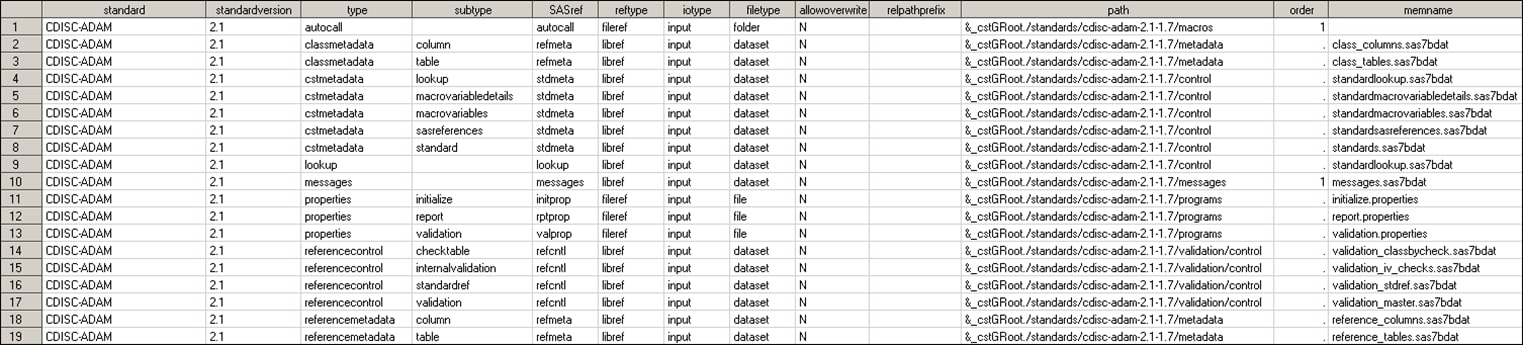
The type and subtype columns
can be used to reference information that the SAS Clinical Standards
Toolkit needs. This information is in the directory structures and
file naming standards used by the customer. A full list of valid types
and subtypes are provided in this document.
The
standards directory
contains subdirectories for each of the standard versions that is
provided with the SAS Clinical Standards Toolkit. In addition, there
are subdirectories for user-customized versions of these standards
and any new user-defined standards. Each subdirectory should be considered
a stand-alone module. This is how the SAS Clinical Standards Toolkit
can keep parallel standards and reduce the need for revalidation.
Within each subdirectory, there might be directories that group the
files, data sets, and housekeeping programs.
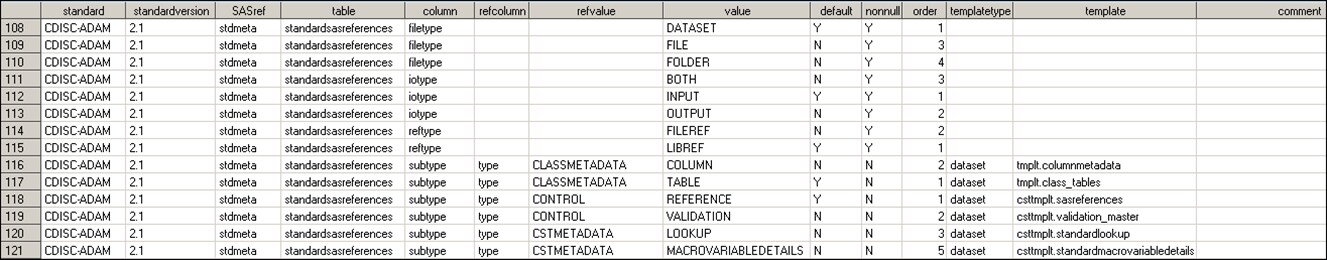
The Standardlookup data
set contains discrete lookup values specific to a SAS Clinical Standards
Toolkit registered standard. It provides specific information for
column values and data set template names. In addition, this data
set is used to perform internal validation of the SAS Clinical Standards
Toolkit.
The following display
shows the entire column list:
Global Standards Library: Metadata Standardlookup Data Set
The availabletransforms.xml
file is for XML-based standards. It defines the location of the XML
schema, the location of the XSL transformation style sheets, and the
import and export locations of XML documents.
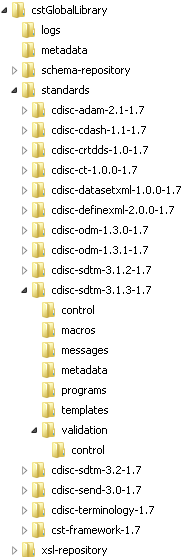
The following display
shows the directory structure for a Microsoft Windows global standards
library with cdisc-sdtm-3.1.3-1.7 expanded:
Directory Structure for a Microsoft Windows Global Standards
Library
The
schema-repository directory
contains XML schema definitions that are used to validate XML files.
Standards that use XML should have their schemas in this directory
so that they can be found. For example, the schema-repository directory
for CDISC CRT-DDS 1.0 as defined in the Standards data set maps to
this location:
global standards library directory/schema-repository/cdisc-crtdds-1.0.0 See Global Standards Library: Metadata Standards Data Set, row 2, schema column.
The
xsl-repository directory
contains files that are used to transform XML files from one format
to another. For example, the default style sheet directory for CDISC
CRT-DDS 1.0 define.xml files created by the SAS Clinical Standards
Toolkit as defined in the Standards data set maps to this location:
global standards library directory/xsl-repository/CRT-DDS/1.0/exportSee Global Standards Library: Metadata Standards Data Set, row 2, exportxsl column.
Copyright © SAS Institute Inc. All Rights Reserved.