Validation of XML-Based Standards
XML Validation
When validating XML-based
standards (such as CDISC ODM, CDISC CT, CDISC CRT-DDS 1.0, and CDISC
Define-XML 2.0, ), the SAS Clinical Standards Toolkit offers two complementary
methodologies.
The
first methodology is described in Compliance Assessment Against a Reference Standard. It relies on the definition of a
master set of validation checks that are specific to the table and
column metadata that define a set of data and on checks that are specific
to the data itself. This method uses SAS files and SAS code to validate
the SAS representation of the XML-based standard. Example checks include
the assessment of foreign key relationships across data sets and value
conformance to a set of expected values.
The second methodology
involves verification that an XML file is valid structurally and syntactically
according to the XML schema for that standard.
The SAS Clinical Standards
Toolkit provides both methodologies to support the validation of CDISC
CRT-DDS 1.0 and CDISC ODM 1.3.0 and 1.3.1 files.
For CDISC Define-XML
2.0 files, SAS Clinical Standards Toolkit supports validation against
an XML schema.
Validating an XML File against an XML Schema: %CSTUTILXMLVALIDATE Macro
The %CSTUTILXMLVALIDATE
macro validates the structure and syntax of an XML file against the
XML schema associated with the XML file. It can be run at any time.
Note: This macro replaces the standard-specific
macros crtdds_xmlvalidate.sas, ct_xmlvalidate.sas, and odm_xmlvalidate.sas.
These macros are deprecated and are deleted in SAS Clinical Standards
Toolkit 1.7. It is recommended that you replace calls to these macros
with a call to the %CSTUTILXMLVALIDATE macro.
The SAS Clinical Standards
Toolkit includes a call to the %CSTUTILXMLVALIDATE macro immediately
following a call to create a specific XML file (for example, the %DEFINE_WRITE
macro to create a CDISC Define-XML 2.0 file). This is typically the
last step of the sample driver program (for example, create_definexml.sas).
If you customize the XML file after it is generated, this macro can
be used to validate the customizations. The SAS Clinical Standards
Toolkit includes a call to the %CSTUTILXMLVALIDATE macro immediately
before a call to read a specific XML file (for example, the crtdds_read
macro to read a CDISC CRT-DDS 1.0 file) from the associated sample
driver program (for example, create_sascrtdds_fromxml.sas).
Here is an example of
a call to the %CSTUTILXMLVALIDATE macro:
%cstutilxmlvalidate(_cstSASReferences=work.sasreferences,_cstLogLevel=info);
In this example, the
%CSTUTILXMLVALIDATE macro is being submitted with a log level of Info.
Note: For more information about
the %CSTUTILXMLVALIDATE macro, see the SAS Clinical Standards Toolkit: Macro API Documentation.
XML schema validation
results are logged using four log-level settings. These log levels
refer to the XML-generated log, not the log that is generated by SAS.
The following table
shows the log levels:
|
Log Level
|
Description
|
|---|---|
|
Info
|
Messages such as the
system properties of the current Java environment and progress messages.
This is the default value.
|
|
Warning
|
Messages that indicate
that there might be an issue with the CRT-DDS document or with the
execution of the validation process.
|
|
Error
|
Messages that indicate
that something in the define.xml document is invalid with respect
to the normal XML schema for CRT-DDS. Or, a non-fatal error has occurred
during processing.
|
|
Fatal Error
|
Messages that indicate
that the XML document could not be processed at all. There are many
causes, including file system access errors, incorrect file paths,
and malformed XML.
|
Each message that is
generated during XML validation is associated with one of these levels.
The level that you choose determines what other messages are generated.
For example, if you choose the Warning level, then all Warning messages
and anything more severe, such as Error and Fatal error messages,
are generated. If you choose the Error level, then only Error and
Fatal Error messages are generated.
Validating the SAS Representation of a CDISC CRT-DDS 1.0 XML File: %CRTDDS_VALIDATE Macro
Overview
The %CRTDDS_VALIDATE
macro supports the first XML validation methodology. This method is
based on SAS and validates the SAS representation of the XML-based
standard.
In the SAS Clinical
Standards Toolkit, CDISC CRT-DDS validation uses the same types of
metadata and the same workflow process that is common to validation
of all data standards. SAS provides a set of validation checks for
CDISC CRT-DDS that are designed to verify the metadata definitions
and values of the 39 data sets that comprise the SAS representation
of the CRT-DDS model. These checks were created by SAS. For
more information about these checks, see Compliance Assessment Against a Reference Standard. Metadata about each check is provided in the Validation
Master data set in
global standards library directory/standards/cdisc-crtdds-1.0-1.7/validation/control.
The %CRTDDS_VALIDATE
macro controls the validation workflow for CRT-DDS. As each check
is processed from the run-time validation check data set, the check
determines the source of the table and column metadata to use. The
reference_tables and reference_columns data sets contain the metadata
for the 39 data sets that comprise the SAS representation for CDISC
CRT-DDS. Unless you make customizations or run-time modifications,
the source metadata source_tables and source_columns data sets contain
the same content as the reference metadata reference_tables and reference_columns
data sets.
If all 39 CRT-DDS tables
contribute information to the define.xml file, then the validation
process can run directly against the reference_tables and reference_columns
data sets. In this case, the Use source data flag in the validation
check data set needs to be set to
N.
However, you are likely to run validation against a subset of the
39 tables. In this case, a source_tables data set that contains the
subset needs to be created from the reference_tables data set. And,
a corresponding source_columns data set needs to be created from the
reference_columns data set. The run-time validation check data set
can contain all of the checks, and Use source data can be set to Y,
which is the default value.
There are no parameters
for the %CRTDDS_VALIDATE macro.
Sample Driver Program: validate_crtdds_data.sas
The validate_crtdds_data.sas
driver program sets up the required environment variables and library
references before a call is made to the %CRTDDS_VALIDATE macro.
The driver program is
located here:
sample study library directory/cdisc-crtdds-1.0–1.7/programsThe SASReferences Data Set
As a part of
each SAS Clinical Standards Toolkit process setup, a valid SASReferences
data set is required. It references the input files that are needed,
the librefs and filenames to use, and the names and locations of data
sets to be created by the process. It can be modified to point to
study-specific files. For
an explanation of the SASReferences data set, see SASReferences File.
In the SASReferences
data set, there are four input file references, one input library
reference, and one output data set reference that are key to the successful
completion of the validation process. Key Components of the SASReferences Data Set for the validate_crtdds_data.sas Driver
Program lists these
files, libraries, and data sets, and they are discussed in separate
sections. In the sample validate_crtdds_data.sas
driver program, these values are set for &studyRootPath and &studyOutputPath:
Note: The &studyRootPath and
&studyOutputPath paths are the same for this driver program. Two
macro variables have been retained to maintain consistency across
the SAS Clinical Standards Toolkit driver programs.
&studyRootPath=sample study library directory/cdisc-crtdds-1.0–1.7&studyOutputPath=sample study library directory/cdisc-crtdds-1.0–1.7|
Metadata Type
|
SAS LIBNAME or Fileref
to Use
|
Reference Type
|
Path
|
Name of File
|
|---|---|---|---|---|
|
Input
|
||||
|
control
|
cntl_s
|
libref
|
&workpath
|
sasreferences.sas7bdat
|
|
control
|
cntl_v
|
libref
|
&studyRootPath/control
|
validation_control.sas7bdat
|
|
sourcemetadata
|
srcmeta
|
libref
|
&studyRootPath/metadata
|
source_tables.sas7bdat
|
|
sourcemetadata
|
srcmeta
|
libref
|
&studyRootPath/metadata
|
source_columns.sas7bdat
|
|
sourcedata
|
srcdata
|
libref
|
&studyRootPath/data
|
|
|
Output
|
||||
|
results
|
results
|
libref
|
&studyOutputPath/results
|
validation_results.sas7bdat
|
Process Inputs
The use of the cntl_s
LIBNAME that points to the &workpath path demonstrates a technique
of documenting the derivation of the SASReferences data set in the
SAS Work library. The driver program initiates the macro variable
&workPath with this statement:
%let workPath=%sysfunc(pathname(work));
In this case, the cntl_s
LIBNAME points to the same directory as the Work LIBNAME. The second
control record points to the validation_control data set (run-time
validation check data set), and is accessed by the cntl_v LIBNAME
statement. This LIBNAME is assigned to the
sample study library directory/cdisc-crtdds-1.0–1.7/control directory.
The sourcemetadata type
references two metadata data sets that describe the table (source_tables)
and column (source_columns) metadata for the 39 data sets that comprise
the SAS representation of the CRT-DDS model. Both data sets are stored
in the same library. In the SAS Clinical Standards Toolkit, this source
metadata is read from the
sample study library directory/cdisc-crtdds-1.0–1.7/metadata directory.
This location is represented in the driver program by the Srcmeta
library name.
The sourcedata type
is the library where the 39 data sets that comprise the SAS representation
of the CRT-DDS model are stored. These are the data sets that are
being validated. In the SAS Clinical Standards Toolkit, this library
is read from the
sample study library directory/cdisc-crtdds-1.0–1.7/data directory.
This location is represented in the driver program by the Srcdata
library name.
Process Outputs
For the SAS Clinical
Standards Toolkit validation processes, the only process outputs that
are generated are the Validation Results and Validation Metrics data
sets. These data sets are described in the following section.
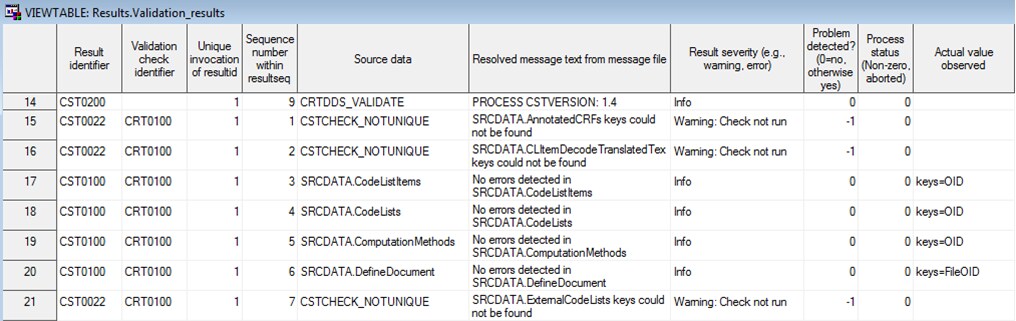
Process Results
When the validate_crtdds_data.sas
driver program finishes running, the validation_results data set is
created in the Results library. The Results data set contains informational,
warning, and error messages that were generated by the driver program.
Reporting of validation process metrics is supported, although it
is not implemented for CDISC CRT-DDS validation.
Example of a CDISC CRT-DDS Results Data Set
Validating the SAS Representation of ODM Files: %ODM_VALIDATE Macro
Overview
The %ODM_VALIDATE macro
supports the second XML validation methodology. This method relies
on the definition of a master set of validation checks that are specific
to the table and column metadata that define a set of data and on
checks that are specific to the data itself. This method uses SAS
files and SAS code to validate the SAS representation of the XML-based
standard.
In the SAS Clinical
Standards Toolkit, CDISC ODM validation uses the same types of metadata
and the same workflow process that is common to validation of all
data standards. SAS provides a set of validation checks for CDISC
ODM that are designed to verify the metadata definitions and values
of the default 66 data sets that comprise the SAS representation of
the ODM model. These checks were created by SAS. For
more information about these checks, see Compliance Assessment Against a Reference Standard. Metadata about each check is provided in the Validation
Master data set in the
global standards library directory/standards/cdisc-odm-1.3.0-1.7/validation/control directory.
The %ODM_VALIDATE macro
controls the validation workflow for ODM. As each check is processed
from the run-time validation check data set, the check determines
the source of the table and column metadata to use. The reference_tables
and reference_columns data sets contain the metadata for the 66 data
sets that comprise the SAS representation for CDISC ODM. Unless you
make customizations or run-time modifications, the source metadata
source_tables and source_columns data sets contain the same content
as the reference metadata reference_tables and reference_columns data
sets.
If all 66 ODM tables
contribute information to the ODM XML file, then the validation process
can run directly against the reference_tables and reference_columns
data sets. In this case, the Use source data flag in the validation
check data set needs to be set to
N.
However, you can choose to run validation against a subset of the
66 tables. In this case, a source_tables data set that contains the
subset needs to be created from the reference_tables data set. And,
a corresponding source_columns data set needs to be created from the
reference_columns data set. The run-time validation check data set
can contain all of the checks, and the Use source data flag can be
set to Y, which is the default
value.
There are no parameters
for the %ODM_VALIDATE macro.
Sample Driver Program: validate_odm_data.sas
The validate_odm_data.sas
driver program sets up the required environment variables and library
references before a call is made to the %ODM_VALIDATE macro.
The driver program is
located here:
sample study library directory/cdisc-odm-1.3.0–1.7/programsThe SASReferences Data Set
As a part of
each SAS Clinical Standards Toolkit process setup, a valid SASReferences
data set is required. It references the input files that are needed,
the librefs and filenames to use, and the names and locations of data
sets to be created by the process. It can be modified to point to
study-specific files. For
an explanation of the SASReferences data set, see SASReferences File.
In the SASReferences
data set, there are three input file references, one input library
reference, and one output data set reference that are key to the successful
completion of the validation process. These
files, libraries, and data sets are listed in Key Components of the SASReferences Data Set for the validate_odm_data.sas Driver
Program, and they are
discussed in separate sections. In the sample
validate_odm_data.sas driver program, these values are set for &studyRootPath
and &studyOutputPath.
Note: The &studyRootPath and
&studyOutputPath paths are the same for this driver program. These
two macro variables have been retained to maintain consistency across
the SAS Clinical Standards Toolkit driver programs.
&studyRootPath=sample study library directory/cdisc-odm-1.3.0–1.7&studyOutputPath=sample study library directory/cdisc-odm-1.3.0–1.7|
Metadata Type
|
LIBNAME or Fileref to
Use
|
Reference Type
|
Path
|
Name of File
|
|---|---|---|---|---|
|
Input
|
||||
|
control
|
cntl_v
|
libref
|
&studyRootPath/control
|
validation_control.sas7bdat
|
|
sourcemetadata
|
srcmeta
|
libref
|
&studyRootPath/metadata
|
source_tables.sas7bdat
|
|
sourcemetadata
|
srcmeta
|
libref
|
&studyRootPath/metadata
|
source_columns.sas7bdat
|
|
sourcedata
|
srcdata
|
libref
|
&studyRootPath/data
|
|
|
Output
|
||||
|
results
|
results
|
libref
|
&studyOutputPath/results
|
validation_results.sas7bdat
|
Process Inputs
The control record points
to the validation_control data set (run-time validation check data
set) data set. It is accessed by the cntl_v LIBNAME statement. This
LIBNAME is assigned to the
sample study library directory/cdisc-odm-1.3.0–1.7/control directory.
The sourcemetadata type
references two metadata data sets that describe the table (source_tables)
and column (source_columns) metadata for the 66 data sets that comprise
the SAS representation of the ODM model. Both data sets are stored
in the same library. In the SAS Clinical Standards Toolkit, this source
metadata is read from the
sample study library directory/cdisc-odm-1.3.0–1.7/metadata directory.
This location is represented in the driver program by the Srcmeta
library name.
The sourcedata type
is the library where the 66 data sets that comprise the SAS representation
of the ODM model are stored. These are the data sets that are being
validated. In the SAS Clinical Standards Toolkit, this library is
read from the
sample study library directory/cdisc-odm-1.3.0–1.7/data directory.
This location is represented in the driver program by the Srcdata
library name.
Process Outputs
For the SAS Clinical
Standards Toolkit validation processes, the only process outputs that
are generated are the Validation Results and Validation Metrics data
sets. These data sets are described in the following section.
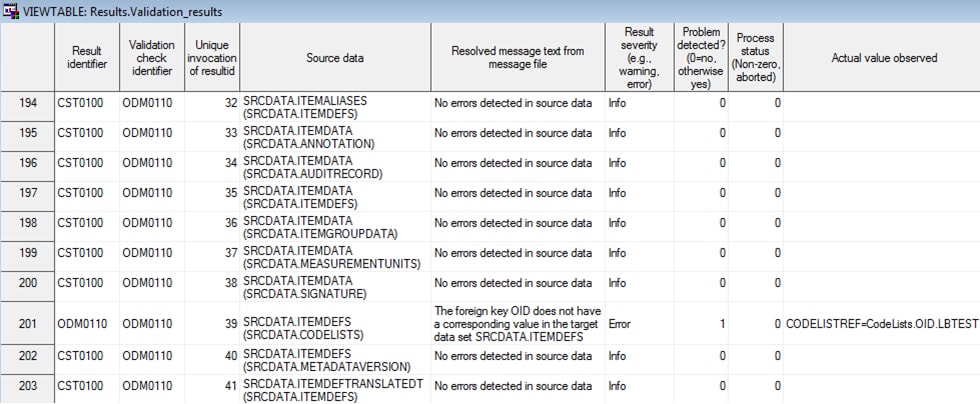
Process Results
When the validate_odm_data
driver program finishes running, the validation_results data set is
created in the Results library. The Results data set contains informational,
warning, and error messages that were generated by the driver program.
Reporting of validation process metrics is supported, although it
is not implemented for CDISC ODM validation.
Example of a CDISC ODM Validation Results Data Set
Copyright © SAS Institute Inc. All Rights Reserved.