Building a SASReferences File
Each SASReferences file
requires content that is specific to its planned use. For example,
a SAS Clinical Standards Toolkit process that creates a define.xml
file requires the specification of XML and recommends the specification
of style sheet information. A SAS Clinical Standards Toolkit process
that validates data against a standard requires the specification
of the validation checks to be run.
The SAS Clinical Standards
Toolkit offers several ways to create a SASReferences file for use
in subsequent processes.
-
Use sample SASReferences files that are provided with the SAS Clinical Standards Toolkit. These sample SASReferences files contain the required and optional contents for specific tasks. For example, the task of validating the functionality of CDISC SDTM 3.1.2 uses the SASReferences file found here in SAS 9.3:
sample study library directory\cdisc-sdtm-3.1.2-1.7\sascstdemodata\controlAn excerpt of this sample SASReferences file is provided in A Sample SASReferences Data Set. -
The SAS Clinical Standards Toolkit provides SASReferences templates for use. These templates are either zero-observation data sets or data sets containing records that must be modified. A SASReferences data set template is located here:
global standards library directory/standards/cst-framework-1.7/templatesThe SAS Clinical Standards Toolkit provides default SASReferences data sets for each supported standard. These default SASReferences data sets contain records that are commonly required for certain SAS Clinical Standards Toolkit tasks (such as validation). However, all records that are required might not be included. Or, all records that are included might not be required for certain tasks. And, SAS librefs, filerefs, paths, and memname values might require modification. For example, see the StandardSASReferences data set found here:global standards library directory/standards/cdisc-sdtm-3.1.2-1.7/control -
The SAS Clinical Standards Toolkit provides the utility macros to build and return many SAS Clinical Standards Toolkit metadata data sets.
-
The %CST_GETSTANDARDSASREFERENCES macro returns the StandardSASReferences data set. (See the file description in Metadata File Descriptions for the specified standard.)
-
The %CST_CREATEDSFROMTEMPLATE macro can be used to return an empty SASReferences data set.
Use of these utility macros is illustrated later in this chapter. -
The
primary function of the SASReferences file is to define the SAS Clinical
Standards Toolkit process inputs and outputs. What information does
the process need to reference? What does the process produce? Where
does the information come from and go? The “what” information
is determined by the use of two SASReferences fields: type and subtype.
The “where” information is determined by path and memname.
The values for all of these fields are restricted for the SAS Clinical
Standards Toolkit to values itemized in the framework Standardlookup
data set found here:
global standards library directory/standards/cst-framework-1.7/control/standardlookup.sas7bdatCustomizing the type
and subtype values in the Standardlookup data set is allowed. Customization
is a prerequisite if you want to use the field values in any SASReferences
data set that is used by the SAS Clinical Standards Toolkit.
The following table lists
and describes the acceptable type and subtype values in the framework
Standardlookup data set:
|
Type
|
Subtype
|
Comments
|
|---|---|---|
|
autocall
|
|
One record for each
library that contains macros to be included in the SAS autocall path.
Typically, this includes one record for each standard that is referenced
in the SASReferences file, excluding the SAS Clinical Standards Toolkit
framework. The framework and cross-standard macros are already included
in the autocall path at product deployment. User-written macros, as
referenced in one or more additional code libraries, require an autocall
record for each library.
|
|
classmetadata
|
column or table
|
Identifies the SAS data
sets (sasref.memname) that contain the column and table metadata for
specific CDISC SDTM template data sets that are used to build standard
SDTM-compliant data sets. This type is provided by default in StandardSASReferences
and is optional.
|
|
cmplib
|
Identifies and sets
the compiled library path to include any user-written and user-referenced
functions. SAS searches the libraries in the order listed until the
desired data set is found.
|
|
|
codemodule
|
Currently not used
and is included only as a placeholder for future ADaM development
within the SAS Clinical Standards Toolkit.
|
|
|
cstmetadata
|
lookup, macrovariabledetails,
macrovariables, sasreferences, standard, or standardsubtypes
|
Identifies the SAS data
set templates that are used for SAS Clinical Standards Toolkit Standards
Library internal validation
|
|
control
|
validation, reference,
or internalvalidation
|
Identifies any run-time
process control file, including the SASReferences data set itself.
(In other words, it is a self-documentation record). For the SAS Clinical
Standards Toolkit validation processes, the Validation Control data
set that specifies the validation checks to be run is identified with
subtype=validation.
|
|
externalxml
|
xml or tlfxml
|
Identifies an external
XML file. Depending on the standard version and the subsequent macro
that is called, this file can be read or written. Using CDISC CRT-DDS
as an example, this type specifies the define.xml file that is created
when the %CRTDDS_WRITE macro is called. When the %CRTDDS_READ macro
is supported, this type identifies the XML file to be read. TLFXML
refers to the tables, listings, and figures XML file that is used
in ADaM 2.1.
|
|
fmtsearch
|
|
Provides a way to build
the format search path for a validation process. The SAS Clinical
Standards Toolkit sets the SAS fmtsearch type based on each record,
specifying a SAS catalog that uses the order=n sequence. This type
is not provided by default in StandardSASReferences, so you must specify
a value. The type=fmtsearch value is optional unless one or more checks
that assess value compliance against a SAS format are to be run.
|
|
globalmetadata
|
sasreferences or standard
|
Identifies the SAS data
set templates that are used for the internal validation of the SAS
Clinical Standards Toolkit global standards library.
|
|
logging
|
transaction
|
Identifies a data set
(transactionlog) that is associated with the SAS Clinical Standards
Toolkit metadata management macros. The data set contains information
about any actions performed on the metadata while using these macros.
|
|
lookup
|
lookup
|
Identifies a data set
(Standardlookup) that is associated with each The SAS Clinical Standards
Toolkit standard that contains valid values for discrete metadata
fields. This type is provided by default in StandardSASReferences
and is required for each standard. For example, the valid values for
type and subtype that are documented in this table have been defined
in one or more SAS Clinical Standards Toolkit Standardlookup data
sets.
|
|
messages
|
|
Identifies one or more
Messages data sets that are associated with each SAS Clinical Standards
Toolkit standard. This type is provided by default in StandardSASReferences.
You must specify value only with user customizations that require
new or modified messages. The SAS Clinical Standards Toolkit populates
the data set that is referenced by the global macro variable &_cstMessages
with all Messages data sets that are included in SASReferences. This
type is required for each standard.
|
|
properties
|
initialize, validation,
or report
|
Initializes a standard
version's required macro variables. Specification in SASReferences
is optional. (These macro variables can be defined with calls to %CST_SETSTANDARDPROPERTIES
or %CST_SETPROPERTIES instead.) Each standard should have at least
one properties (initialize) file. Each standard can have any additional
files that are needed. A subtype=validation value is specific to SAS
Clinical Standards Toolkit validation processes.
|
|
referencecontrol
|
validation, standardref,
checktable, or internalvalidation
|
If subtype=validation,
then the value identifies the standard-supplied master superset of
supported validation checks. Although this is key metadata, it is
not typically referenced at run time and does not need to be included.
It is the Validation Control file that is identified with type=control
and subtype=validation that must be included.
If subtype=standardref,
then the value identifies an optional data set that contains a list
of references that provide the basis for each validation check that
is included in the subtype=validation data set.
|
|
referencecterm
|
|
Identifies a SAS data
set (sasref.memname) that most often contains controlled terminology,
as opposed to a SAS format containing controlled terminology (for
example, medDRA). The type=referencecterm value is optional unless
one or more checks are to be run that assess value compliance against
a SAS data set.
|
|
referencemetadata
|
column or table
|
Identifies the SAS data
sets (sasref.memname) that contain the column and table metadata for
a standard version. This type is provided by default in StandardSASReferences,
so you must specify a value only to override the default for the standard.
Records for both subtypes are required.
|
|
referencexml
|
stylesheet, map, tlfxml,
datamap, or metamap
|
If subtype=stylesheet,
then this value identifies the directory and filename of an XML style
sheet. In the production of CDISC CRT-DDS XML files, this value should
point to the style sheet to be copied into the directory with the
XML file.
If subtype=map, then
this value identifies the persisted location of a SAS XML map file.
The SAS XML map file reads the Work cube.xml file generated by the
SAS Clinical Standards Toolkit that translates an XML file into the
SAS representation of the XML-based standard (such as CDISC CRT-DDS
and CDISC ODM).
If subtype=metamap or
subtype=datamap, then this value identifies the map file that supports
reading metadata or data from XML files.
|
|
report
|
library or outputfile
|
Specifies the storage
location of the SAS Clinical Standards Toolkit process reports. If
a single, specific report is referenced, then it can be specified
with a subtype of outputfile, a valid path, and valid memname values.
If the process produces multiple reports, then a subtype of library
is used with a valid path to the directory or folder. In the latter
case, default report names as defined in the code are used.
|
|
results
|
analysis or results
or validationresults, metrics or validationmetrics
|
Specifies the storage
location of the Results and Metrics data sets that are generated by
the SAS Clinical Standards Toolkit process. The Metrics data set is
specific to the SAS Clinical Standards Toolkit validation processes
and is optional depending on property settings. A
results/validationresults record
is required.
Note: Analysis has been added
for the SAS Clinical Standards Toolkit, but it is not used.
|
|
resultspackage
|
xml or log
|
This type is not used
in the SAS Clinical Standards Toolkit. This type bundles a set of
process inputs and outputs together for later access.
|
|
sourcedata
|
|
Defines the folder location
of the data for a specific study. This type is required for validation
processes if one or more checks are to be run that access a specific
source data domain.
|
|
sourcemetadata
|
analyses, analysisresult,
column, document, value, table, study, codelist, or itemgroup
|
Identifies the SAS data
sets (sasref.memname) that contain the column, document, analyses,
analysis result, value (for value level metadata), codelist, itemgroup,
study, and table metadata for a study or set of source data. This
type is not provided by default in StandardSASReferences, so you must
specify a value. Records for both subtypes are required.
|
|
standardmetadata
|
attribute or element
|
Identifies the SAS data
set templates for valid_attributes and valid_elements when validating
ODM files.
|
|
standards
|
registeredstandards
or registeredsasreferences
|
Identifies the template
for the registered Standards and SASReferences data sets, respectively.
This value is used by the framework when the global metadata library
is created. This type is not used in post-deployment processes.
|
|
studymetadata
|
analyses, analysisresult,
codelist, column, document, value, table, study, or itemgroup
|
Identifies the SAS data
sets (sasref.memname) that contain the table, column, codelists, document,
analyses, analysis result, value (for value level metadata), study,
and itemgroup metadata for a study. This type is not provided by default
in StandardSASReferences, so you must specify a value. Records for
both subtypes are required.
|
|
targetdata
|
Defines the location
of the data to be derived for a specific standard. For example, for
CDISC CTR-DDS, the %CRTDDS_READ macro derives a set of CRT-DDS data
sets from the referenced define.xml file. This type is optional.
|
|
|
targetmetadata
|
analyses, analysisresult,
document, value, column, table, study, codelist, or itemgroup
|
Identifies the SAS data
sets (sasref.memname) that contain the analyses, analysis result,
document, value (for value level metadata), codelist, itemgroup, study,
column, table, and study metadata to be derived for a specific standard.
For example, for CDISC CRT-DDS, the %CRTDDS_READ macro derives files
that describe metadata about the targetdata data sets that are derived
from the referenced define.xml file. If this type is used, then a
record for each subtype is required.
|
|
template
|
Identifies the library
for metadata template data sets that are used to generate table shells.
|
|
|
transport
|
This type is not used
in the SAS Clinical Standards Toolkit. This type identifies a library
of SAS transport files that are optionally referenced by a define.xml
file.
|
Every instance of the
SASReferences file does not require a specific path and filename.
At the beginning of this section, a call to this macro was described:
%cst_getstandardsasreferences(_cstStandard=CST-FRAMEWORK, _cstStandardVersion=1.2,_cstOutputDS=sasreferences);
The following display
shows that this macro call produces this SASReferences file:
Standard SASReferences File for CST-FRAMEWORK
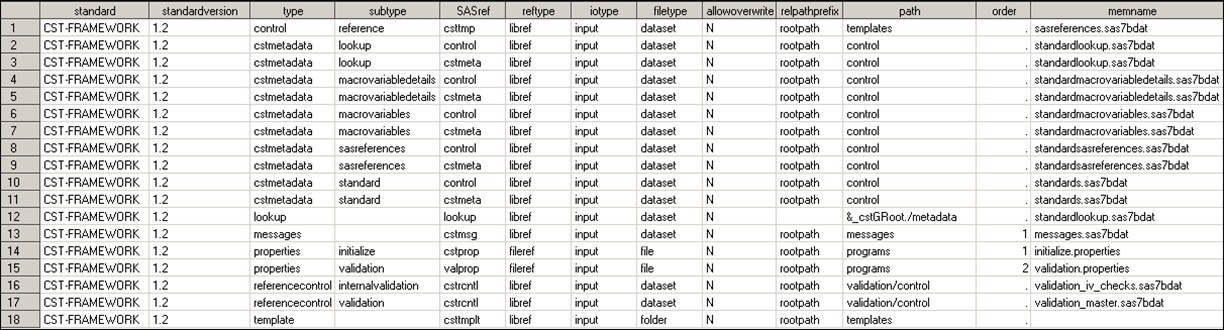
The SASref field,
with values of cstmeta and control,
points to the same path field value. The control SASref
was retained to ensure backward compatibility with past releases.
Standard SASReferences for CDISC SDTM shows the information returned by
this call to %CST_GETSTANDARDSASREFERENCES for the CDISC SDTM standard:
%cst_getstandardsasreferences(_cstStandard=CDISC-SDTM, _cstOutputDS=sasreferences);
Standard SASReferences for CDISC SDTM
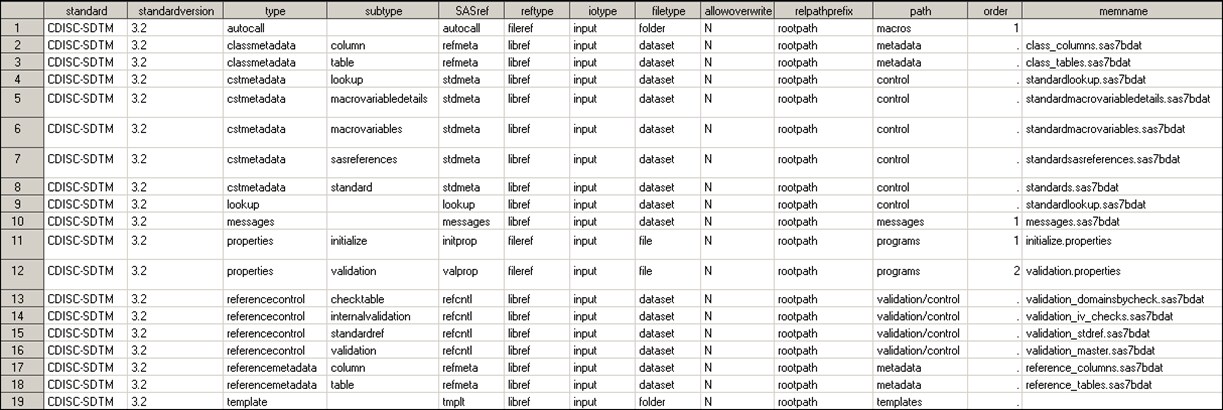
A comparison
of Standard SASReferences File for CST-FRAMEWORK and Standard SASReferences for CDISC SDTM shows little similarity in the record types and no overlap
in references to specific files. The target inputs and outputs for
CDISC SDTM are more focused on the task (for example, validating SDTM
domains). The SAS Clinical Standards Toolkit validation processes
require specification of a comparative reference standard. Here, there
are references to a standard-specific macro library (autocall), Messages
data set, and properties files. Unique SASref values by type are provided,
pointing to distinct files and folders in the global standards library.
Consider an actual SASReferences
file built to support CDISC SDTM 3.1.2 validation. The task of validating
the functionality of CDISC SDTM 3.1.2 uses the SASReferences file
here in SAS 9.3 and SAS 9.4:
sample study library directory\cdisc-sdtm-3.1.2-1.7\sascstdemodata\controlThe following display
shows the complete contents of the SASReferences file:
Sample SASReferences File for CDISC SDTM Validation
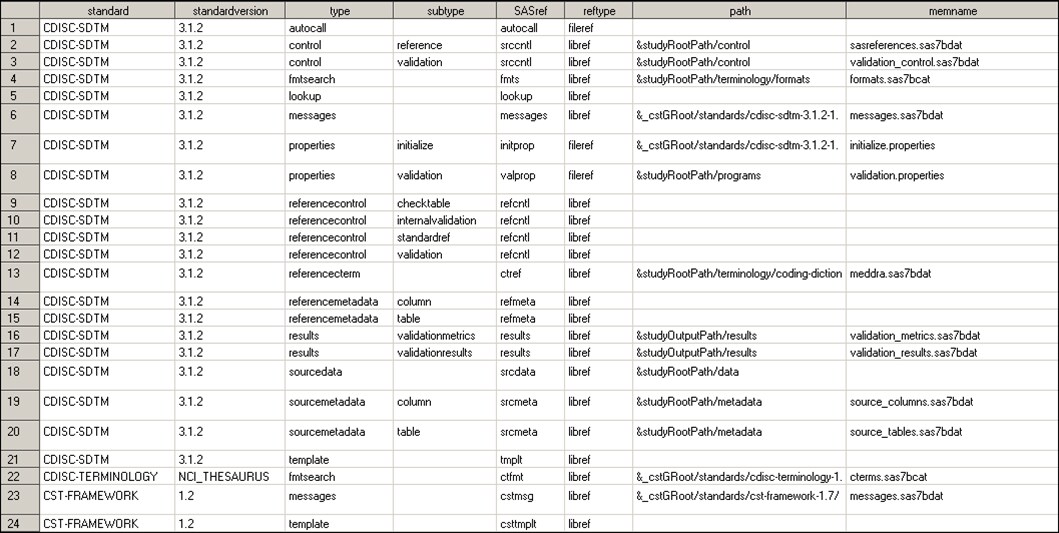
|
Lines
|
Comment
|
|---|---|
|
1
|
Instructs the SAS Clinical
Standards Toolkit to add any SDTM-specific macros to the autocall
path.
|
|
2
|
Documents the name and
location of this file. This information is used in the sample reports
that are discussed in this document.
|
|
3
|
Points to the set of
validation checks to be run in this validation assessment. The framework
default values for SASref, path, and memname have been overridden.
|
|
4, 22
|
Two standards are referenced
to create a format search path. Line 4 references the SDTM study-specific
formats catalog. Line 22 references the more general CDISC Controlled
Terminology cterms catalog. The precedence is set by the order column.
|
|
6, 23
|
These records are identical
to the CST-FRAMEWORK and CDISC-SDTM StandardSASReferences records.
|
|
7
|
Illustrates the call
to a standard-specific properties file that is used to initialize
a global macro variable that is specific to that standard. Referencing
a standard-specific properties files in the SASReferences data set
is recommended. The call to the CST-FRAMEWORK initialize.properties
file is a prerequisite setup step outside of SASReferences and performed
before processing SASReferences.
|
|
8
|
The validation properties
path has been modified to point to a location in the study hierarchy,
rather than to the global standards library that is defined in the
StandardSASReferences file.
|
|
9–12, 14–15,
21, 24
|
Points to
the reference standard for CDISC SDTM 3.1.2, but unlike the template
defaults in Standard SASReferences for CDISC SDTM, path and memname are blank. Leaving
them blank tells the SAS Clinical Standards Toolkit to look in the
CDISC SDTM 3.1.2 StandardSASReferences file and use the defaults for
that standard and version. This convention facilitates portability
of the data set by doing a run-time lookup for the current information. The lookup
results in the inclusion of the path and memname values as defined
in Standard SASReferences for CDISC SDTM.
|
|
13
|
References a medDRA
data set that is maintained in the study-specific hierarchy. A more
common implementation might reference a non-study-specific coding
dictionary.
|
|
16–17
|
Specifies that process
results are to be stored in a location in the study hierarchy.
|
|
18
|
This is a type that
is not in the template files (StandardSASReferences). It defines the
location of the study (source) data. The use of &studyRootPath,
coupled with the assumption of a fixed-folder hierarchy, enables portability
across studies. The memname value is not relevant for a library of
SAS data sets.
|
|
19–20
|
These values follow
the style used in line 18 for source data. The same SASref is used
for multiple subtypes in a single type because the subtypes reference
two differently named SAS data sets from the same folder.
|
An alternative way to
build the SASReferences file is to use the %CST_CREATEDSFROMTEMPLATE
utility macro.
%cst_createdsfromtemplate(_cstStandard=CST-FRAMEWORK,_cstType=control, _cstSubType=reference,_cstOutputDS=work.sasreferences); proc sql; insert into work.sasreferences values(CST-FRAMEWORK 1.2 messages messages libref 1 ); . . . quit;
This macro copies the
template. New records can be added various ways, including the previous
PROC SQL technique. There is no requirement that the SASReferences
file has to live outside the SAS Work area and be kept beyond the
SAS Clinical Standards Toolkit process. However, these are best practices
that enable future capabilities such as process reruns and reporting.
Copyright © SAS Institute Inc. All Rights Reserved.