Standards
The Standards data set
is used by the SAS Clinical Standards Toolkit framework to store information
about a standard version. All standards that are provided by SAS,
and standards that you might want to add are defined in the global
standards library in the metadata/standards data set. All calls to
the %cst_registerstandard macro that are described in Chapter 2 interact
directly with the metadata/standards data set.
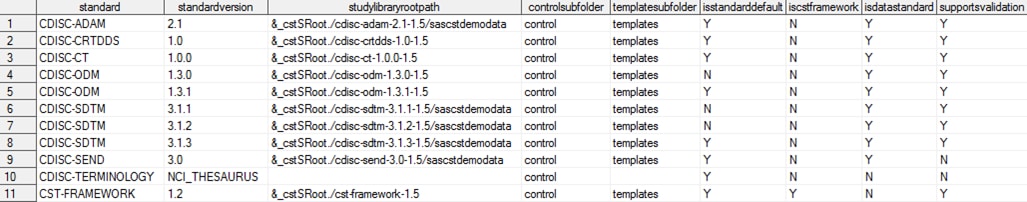
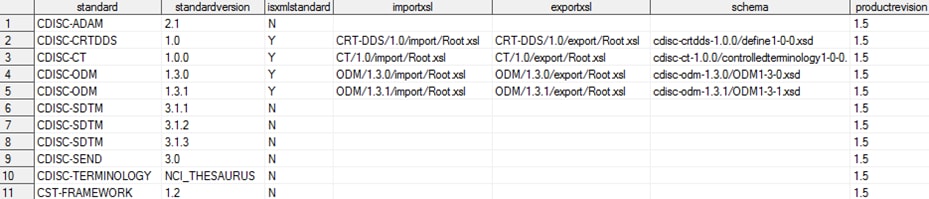
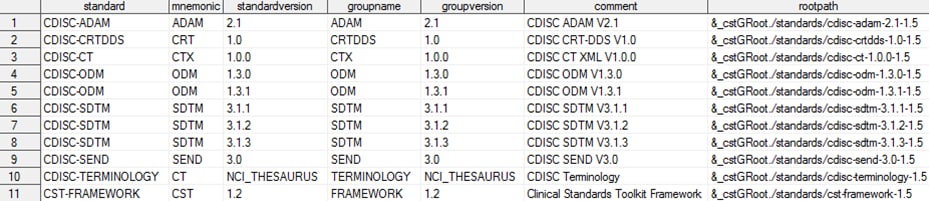
The global standards
library data set contains these records, which are provided with the
SAS Clinical Standards Toolkit 1.5 (the columns are continued in the
subsequent two images):
Metadata/Standards Data Set Content in the Global Standards
Library
The
&_cstGRoot in
the rootpath column maps to the global standards library directory that
is set by calling the cstutil_setcstgroot macro.
&_cstSRoot
in the studylibraryrootpath column maps to the sample study library directory
that is set by calling the cstutil_setcstsroot macro.
Copyright © SAS Institute Inc. All rights reserved.