By default,
SAS Clinical Standards Toolkit provides a Messages data set for all
SAS Clinical Standards Toolkit framework standards and for each data
standard provided by SAS. Each Messages data set includes a list of
codes and associated text that are specific to each standard. In some
cases, actions such as validation are used to report process results.
The structure of all the message files is described in the following
table.
Messages Data Set Structure
|
|
|
|
|
|
|
|
The message ID. SAS
Clinical Standards Toolkit has adopted a naming convention matching
each standard. The resultid values are prefixed with an up to 4-character
prefix (CST for framework messaging; CDISC examples: ODM, SDTM, ADAM,
and CRT). By convention, the prefix matches the mnemonic field in the Standards data set in <global standards library directory>/metadata. This prefix is followed by a 4-digit numeric that is unique within
the standard (for example, SDTM1234). Users can use any naming convention
limited to 8 characters. For CDISC standards supporting validation,
the resultid should match the checkid from the Validation Master data
set for standard records that support validation.
|
|
|
|
|
A specific version of
a standard. This value must match one of the standard versions that
is associated with a registered standard. This value must also match
the standardversion field in the SASReferences
data set. The only exception to this rule is that *** can be used
to signify that the check applies to all supported versions of the
standard (for example, 3.1.1, 1.0, ***). If a subsequent version of
the standard is released, then *** would be applicable if the check
is valid for the new version.
|
|
|
|
|
A string that identifies
the source of the message. This string is used to provide source-specific
messages generated within the SAS Clinical Standards Toolkit. CDISC
examples include Janus, OpenCDISC, SAS, and WebSDM. This field can
contain any user-defined value.
|
|
|
|
|
A reference identifier
for this message from the checksource.
|
|
|
|
|
The severity as assigned
by checksource. This value is mapped
to the following standardized values: Note (Low), Warning (Medium),
Error (High). A value is expected, although it is not technically
required. It is used in reporting.
|
|
|
|
|
A full description of
the validation check that is associated with checksource if the source is external to SAS Clinical Standards Toolkit. If checksource is set to CST, then this field is null.
|
|
|
|
|
The default message
text to be written to the Results data set. This field can contain
0, 1, or 2 parameters. By convention, parameters are _cstParm1 and
_cstParm2, but any _cst prefix parameter is recognized. The fully
resolved messagetext that includes
substituted parameter values is written to the Results data set.
|
|
|
|
|
The message parameter1
(_cstParm1) default value. If the code using the message does not
provide a parameter value, then this default value is used. This column
can be null.
|
|
|
|
|
The message parameter2
(_cstParm2) default value. If the code using the message does not
provide a parameter value, then this default value is used. This column
can be null.
|
|
|
|
|
Any additional information
that explains the message.
|
|
The Messages
data set that supports the SAS Clinical Standards Toolkit framework
can be found at:
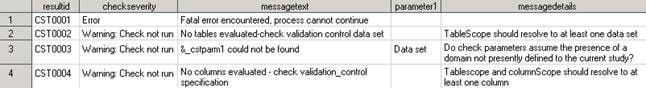
<global standards library directory>/standards/cst-framework-1.3/messages/messages.sas7bdatThe following display
provides an excerpt of records and columns from the SAS Clinical Standards
Toolkit framework Messages data set.
Framework Messages Data Set
For
more information about messages supporting the SAS Clinical Standards
Toolkit framework, see
ResultIDs and Associated Message Text. Other message-type data sets that
support non-framework standards are described in this document.