Special Topic: How SAS Clinical Standards Toolkit Interprets Validation Check Metadata
Overview
Four Validation
Master metadata fields are key to how the SAS Clinical Standards Toolkit
processes source data and source metadata: usesourcemetadata, tablescope,
columnscope, and codelogic.
The SAS
Clinical Standards Toolkit uses usesourcemetadata to point to the
correct metadata. If usesourcemetadata is set to Y, then the SAS Clinical
Standards Toolkit knows that the source metadata (source_tables and
source_columns) is to be used to derive the domains and columns to
be evaluated for compliance to the standard. If usesourcemetadata
is set to N, reference metadata (reference_tables and reference_columns)
is to be used.
The SAS
Clinical Standards Toolkit uses the tablescope and columnscope values
to build the work._csttablemetadata and work._cstcolumnmetadata data
sets. Based on the values of these fields, the SAS Clinical Standards
Toolkit creates a subset of source metadata or reference metadata
that represents the union of tablescope and columnscope. The SAS Clinical
Standards Toolkit builds columns specified in columnscope that also
exist in the tables specified in tablescope.
Case Study 1: CDISC SDTM Check SDTM0604
In this
case study, whether the sequence numbers (**SEQ) used in various domains
are consecutively incremented beginning at 1 for each USUBJID is determined.
There
are specific values to assign to usesourcemetadata, tablescope, and
columnscope to set up a proper test of sequence numbers. First, you
want to include the domains you actually have (that is, source data
and metadata). So, set usesourcemetadata to Y. Next, you want to test
all domains that contain sequence numbers. So, set tablescope to _ALL_.
Because each domain uses a domain-specific name for sequence number,
set columnscope to "**SEQ".
%let _cstLastKey=%scan(%quote(&_cstSubjectKeys),-1,","); data work._cstproblems (drop=count); set &_cstDSName (keep=&_cstDSKeys &_cstColumn); by &_cstDSKeys; if first.&_cstLastKey then count=1; else count+1; if &_cstcolumn ne count then output; run;
The following
five macro variables are used in this code. They are representative
of variables set in many of the check macros before calling code logic.
See each validation check macro for local macro variables available
to code logic.
Processing
based on Validation Master metadata fields results in records being
added to work._cstproblems for any record that does not match the
record counter within the subject.
However,
there are two records in the Validation Master check data set for
the CDISC SDTM check SDTM0604. The tablescope and columnscope settings
for each record differ from the previous description. The CDISC SDTM
TS (Trial Summary) domain does not contain the subject key USUBJID.
The previous code logic does run against the TS domain without failing.
(But, the SAS log indicates a problem:
NOTE: Variable
first.USUBJID is uninitialized.). A better solution is
offered in the Validation Master check data set with the two records.
Case Study 2: CDISC SDTM Check SDTM0623
In this
case study, whether the values for standard units (**STRESU) are consistent
within each test code (**TESTCD) across all records in the CDISC SDTM
findings domains is determined.
You want
to include the domains you actually have (that is, source data and
metadata). So, set usesourcemetadata to Y. Next, you want to test
all findings domains, which typically contain these two domain columns
(**STRESU and **TESTCD). So, you might want to set tablescope to CLASS:FINDINGS.
Because you want to compare two columns in each domain, set columnscope
to [**TESTCD][**STRESU]. For more information about tablescope and
columnscope syntax, see Column Descriptions of the Validation Master Data Set.)
data work._cstunique;
set work._cstunique;
by &_cstColumn1 &_cstColumn2;
if first.&_cstColumn1=0 or last.&_cstColumn1=0 then _checkError=1;
run;
proc sort data=&_cstDSName out=&_cstclds;
by &_cstColumn1 &_cstColumn2;
run;
data work._cstuniqueerrors;
merge work._cstunique (where=(_checkerror=1) in=un)
&_cstclds (in=ds);
by &_cstColumn1 &_cstColumn2;
if un and ds and first.&_cstColumn2;
run;
This case
study shows how the SAS Clinical Standards Toolkit uses local macro
variables for column comparisons. The columnscope syntax [**TESTCD][**STRESU]
tells the SAS Clinical Standards Toolkit to create two sublists. The
first sublist is for all TESTCD columns, and the second is for all
STRESU columns. These are referenced as &_cstColumn1 and &_cstColumn2
in code logic, respectively.
In this
case, the validation check macro that calls and interprets code logic
output (cstcheck_notunique)
reports all work._cstuniqueerrors records as failing this instance
of CDISC SDTM check SDTM0623.
It fails
now because of the way it has been configured. The following sections
shows how to solve the problem. The generated Results data set contains
the following excerpt:
The actual and resultdetails values
give clues about the problem. The SAS Clinical Standards Toolkit resolves
the columnscope sublist [**TESTCD] to five columns. It resolves the
sublist [**STRESU] to four columns. The SAS Clinical Standards Toolkit
column comparisons require sublists of equal length so that valid
comparisons can be made. There appears to be a findings domain that
has TESTCD, but not STRESU. In this case, the domain IE does not have
the column IESTRESU. Attempting to compare IETESTCD with LBSTRESU
is not the intention.
Tablescope
and columnscope syntax supports wildcarding and addition and subtraction
operators. However, this flexible functionality is not required. You
can submit explicit table and column references. CDISC SDTM check
SDTM0623 could be defined in the Validation Master data set as the
following:
Both of
the above definitions will run correctly, but do not yet match the
record metadata for SDTM0623 in the SAS Validation Master data set:
The reason
LB is excluded from tablescope is because CDISC SDTM
check SDTM0631 is a specific test of these LB domain columns (the
Validation Master checksource and sourceid fields show SDTM0631 to be an implementation
of the WebSDM check IR5006). SDTM0623 is simply a generalization of
SDTM0631 to include all findings domains. There is no reason to redundantly
test LB.
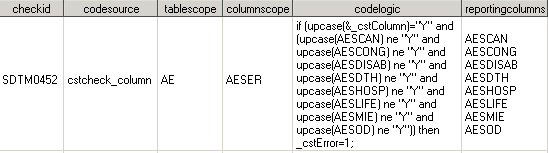
Case Study 3: CDISC SDTM Check SDTM0452
In the
CDISC SDTM Adverse Events (AE) domain, AE is defined as serious (AESER="Y"),
but none of the serious qualifier columns has been set to "Y". (For
example, AE involves cancer (AESCAN).)
This case
study is an example of a validation check with a specific implementation
using hardcoded column references with no wildcarding.
The tablescope and columnscope fields
specify a single table and column. The reportingcolumns value is used by the cstcheck_column check macro to include these
columns for check processing and to report the column values in the
Results data set actual field.
But what
happens if, in your source study, you did not collect all eight of
the qualifier columns expected by the check metadata? By default,
an error message such as the following is written to the SAS log:
-
Do not run the check at all. The check can be removed from the run-time Validation Control data set. Disabling a check can be done by removing it from the Validation Master data set or by setting the checkstatus flag to some value other than 1. (This assumes that the process that you use to extract checks into the run-time Validation Control data set references the checkstatus field).
-
Add missing (all null) columns to your AE domain, and include those columns in the source_columns metadata. (In this example, these columns are defined in the CDISC SDTM standard as permissible columns.) While this solves the immediate problem, it is not a recommended best practice, which is to exclude all-null columns defined as permissible. In fact, adding all-null columns triggers the reporting of another check (SDTM0605).
-
Can columnscope be modified to include the qualifier columns that you do have? The answer is no. The columnscope field defines the scope of the primary columns to be tested. The check macro code loops through the resolved set of columns from the columnscope field, and it evaluates the validity of each column. In this case, you do not want to test each of the qualifier columns against each other.
-
Modify the current check record metadata with updated codelogic and reportingcolumns values. This seems simple enough. Remove the columns that are not collected in the source study. Your best practices control this action. For example:You should consider the implications of modifying existing checks with regard to future product updates and synchronization of changes. For more information, see Special Topic: Validation Customization.