Maintenance Usage Scenarios
Overview
The following
sections describe usage scenarios that the framework accommodates.
Code that is required to complete the usage scenario included in each
section. All macros that are provided in the usage scenarios can be
found in the primary SAS Clinical Data Standards Toolkit autocall
path:
For complete
macro documentation, see Macro Application Programming Interface.
Registering a New Version of a Standard
The following
code defines and registers a new standard. The code can also be used
to register a new version of an existing standard.
/*
Step 1. Ensure that the macro variable pointing to the global standards
library exists.
*/
%cstutil_setcstgroot;
/*
Step 2. Register the standard with the Toolkit global standards
library
*/
%cst_registerStandard(
_cstRootPath=%nrstr(&_cstGRoot./standards/myStandard),
_cstControlSubPath=control,
_cstStdDSName=standards,
_cstStdSASRefsDSName=StandardSASReferences);
The
_cstRootPath parameter uses %nrstr(&_cstGroot) so that the &_cstGroot is registered
as a macro variable. This specification allows the global standards
library to be moved or copied without reregistering the full path
of the new standard.
When defining and registering
a new standard, you should evaluate which of the metadata files described
in Common Framework Metadata should be provided to support new standard functionality.
For example:
For more
information about the metadata files that support SAS Clinical Standards
Toolkit, see Metadata File Descriptions.You can define new metadata types. These new
metadata types should be documented in the standard-specific StandardSASReferences
and Standardlookup data sets, and in the SAS Clinical Standards Toolkit
framework Standardlookup data set.
Setting the Default Version for a Standard
When multiple
versions of a standard exist, the first version that is installed
is set as the default. The default version is used when multiple versions
of a standard have been registered, and a specific version is not
provided in a macro call or in a SASReferences file. The following
code modifies the default version of a specific standard:
%cst_setStandardVersionDefault(
_cstStandard=CDISC-SDTM
,_cstStandardVersion=3.1.1
);
Unregistering a Standard Version
If a standard
becomes obsolete and needs to be unregistered, then use the framework
to do this. Unregistering a standard might be needed during the development
of a custom standard. The following macro call unregisters the CDISC
SDTM 3.1.1 standard, removes it from the global standards library
metadata Standards data set, and removes all records for 3.1.1 from
the StandardSASReferences data set:
%cst_unregisterStandard(
_cstStandard=CDISC-SDTM
,_cstStandardVersion=3.1.1
);Unregistering an Old Version of a Standard, and then Registering a New Version of a Standard
Suppose
SAS Clinical Standards Toolkit 1.2 is currently installed and used.
SAS Clinical Standards Toolkit 1.3 is released. You want the product
updates for a standard version. In the following steps, the CDISC
SDTM standard is used as an example. However, the steps apply to all
other standard versions. You want to set version 3.1.2 as the default
version for the CDISC SDTM standard. The SAS Clinical Standards Toolkit
installation process does not do this automatically because you might
have made updates to the SAS Clinical Standards Toolkit 1.2 code base
or metadata that you want to preserve. Or, you might want to test
the SAS Clinical Standards Toolkit 1.3 CDISC SDTM 3.1.2 implementation
before declaring it the new default version.
Step 1:
Confirm that multiple versions of the standard are available. Confirm
that registration of a new version is needed.
-
Confirm which revision of the standard-version is currently in use.
-
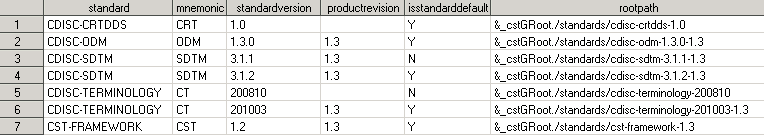
Open the Standards data set in the library, and confirm that the older version is the one being used. The following display shows that the registered version CDISC SDTM 3.1.1 has no product revision value that indicates that it is the original version that was shipped with SAS Clinical Standards Toolkit 1.2. It is defined as the default version for the CDISC SDTM standard.
Step 2:
Register the updated CDISC SDTM 3.1.1 metadata in the global standards
library to use the SAS Clinical Standards Toolkit 1.3.
-
%cstutil_setcstgroot; /* Set the framework properties used for the uninstall */ %cst_setStandardProperties( _cstStandard=CST-FRAMEWORK, _cstSubType=initialize ); /* If the version to be replaced is the default, you must make another version the default. In this case, this is the desired final outcome anyway. */ %cst_setStandardVersionDefault( _cstStandard=CDISC-SDTM ,_cstStandardVersion=3.1.2 ); /* Unregister the standard */ %cst_unregisterStandard( _cstStandard=CDISC-SDTM ,_cstStandardVersion=3.1.1 );