Monitoring the Statuses of Domains
Monitor the Progress of a Study or Submission
To monitor the progress
of a study or submission, perform the following steps:
-
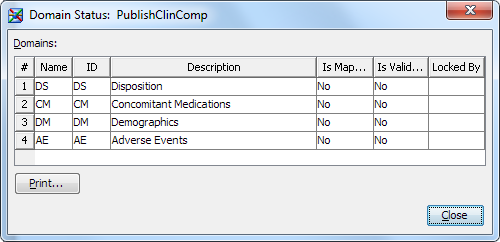
The Domain Status dialog box appears.The Domains table reports the following information:
-
the name of the domain
-
the ID of the domain
-
the description of the domain
-
whether the domain contains defined mapping jobs (that is, the domain contains a job where the domain is a target)
-
whether the domain contains defined compliance jobs (that is, the domain is selected to be validated)
-
whether the domain is locked and by whom
-
Copyright © SAS Institute Inc. All rights reserved.